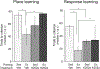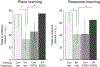Use it and boost it with physical and mental activity - PubMed (original) (raw)
Review
Use it and boost it with physical and mental activity
Donna L Korol et al. Hippocampus. 2013 Nov.
Abstract
One of the now classic tenets of neuroscience is that the brain retains a substantial amount of structural and functional plasticity throughout adulthood and old age. Enriching experiences that stimulate physical and mental activity produce robust changes in subsequent behaviors, including learning and memory, that tap a wide range of neural systems. In this article, we review evidence for cognitive priming with physical and mental exercise through a memory systems lens and present brain-derived neurotrophic factor (BDNF) signaling as one candidate neural mechanism for experience-dependent modulation of learning and memory. We highlight our recent findings showing that priming with voluntary exercise or with spontaneous alternation, a working memory task, enhances new learning of hippocampus-sensitive place, or striatum-sensitive response tasks. Blocking BDNF signaling with infusions of a BDNF receptor inhibitor into hippocampus or striatum just before training on place or response tasks, respectively, abrogated the benefits of priming regardless of the type of priming experience. These results suggest that enhanced BDNF signaling during learning may itself produce the cognitive benefits afforded by prior physical or mental activity.
Keywords: BDNF; learning strategy; memory systems; plasticity; voluntary exercise.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
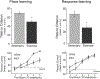
FIGURE 1.
Three weeks of exercise in adult male rats improved both place and response learning. Trials to reach criterion (upper panels) were significantly reduced for both tasks. Exercising rats showed higher percent correct (lower panels) in the second half of place training but higher percent correct in the first half of training for the response task. *P < 0.05.
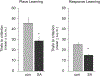
FIGURE 2.
Testing on a 4-arm spontaneous alternation working memory task for 20 min improved both place and response learning in male rats. Trials to reach criterion were significantly lower for rats that had alternation testing 1 h before place or response training (dark bars) than for untested control rats (gray bars). *P < 0.05.
FIGURE 3.
Levels of mature BDNF (mBDNF) in the hippocampus (left graphs) and striatum (right graphs) following 3 weeks of exercise (A) or 20 min of spontaneous alternation testing (B). mBDNF in the hippocampus rose dramatically after alternation testing but not 3 weeks of exercise. Priming with exercise or cognitive testing substantially increased mBDNF in striatum compared to control levels. *P < 0.05.
FIGURE 4.
Effects of pretraining K252a or vehicle control (Veh) infusions on exercise-induced enhancements in place (left panel) and response (right panel) learning. Intrahippocampal K252a fully reversed place learning enhancements seen following 3 weeks of voluntary exercise. Intrastriatal K252a in exercising rats attenuated enhancements and in sedentary (Sed) rats produced enhancements in response learning. *P < 0.05.
FIGURE 5.
Effects of pretraining K252a or vehicle control (Veh) infusions on spontaneous alternation (SA)-induced enhancements in place (left panel) and response (right panel) learning. Compared to no testing (Con), 20 min of SA testing produced enhancements in place learning that were fully reversed by intrahippocampal K252a infusions. Intrastriatal K252a infusions also blocked the enhancements in response learning seen by SA testing. *P < 0.05.
FIGURE 6.
A schematic of our working model for the effects of BDNF signaling through its cognate TrkB receptor. BDNF signaling that produces rapid or acute activating actions are likely to promote faster learning. Note also that downstream effects of BDNF that reflect more durable trophic actions of TrkB signaling, including increased expression of BDNF itself, may mediate or modulate the plasticity involved in priming.
Similar articles
- Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I.
Liu YF, Chen HI, Wu CL, Kuo YM, Yu L, Huang AM, Wu FS, Chuang JI, Jen CJ. Liu YF, et al. J Physiol. 2009 Jul 1;587(Pt 13):3221-31. doi: 10.1113/jphysiol.2009.173088. Epub 2009 May 18. J Physiol. 2009. PMID: 19451201 Free PMC article. - Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling.
Aguiar AS Jr, Castro AA, Moreira EL, Glaser V, Santos AR, Tasca CI, Latini A, Prediger RD. Aguiar AS Jr, et al. Mech Ageing Dev. 2011 Nov-Dec;132(11-12):560-7. doi: 10.1016/j.mad.2011.09.005. Epub 2011 Oct 1. Mech Ageing Dev. 2011. PMID: 21983475 - Late-Life Environmental Enrichment Induces Acetylation Events and Nuclear Factor κB-Dependent Regulations in the Hippocampus of Aged Rats Showing Improved Plasticity and Learning.
Neidl R, Schneider A, Bousiges O, Majchrzak M, Barbelivien A, de Vasconcelos AP, Dorgans K, Doussau F, Loeffler JP, Cassel JC, Boutillier AL. Neidl R, et al. J Neurosci. 2016 Apr 13;36(15):4351-61. doi: 10.1523/JNEUROSCI.3239-15.2016. J Neurosci. 2016. PMID: 27076430 Free PMC article. - Brain-derived neurotrophic factor in amygdala-dependent learning.
Rattiner LM, Davis M, Ressler KJ. Rattiner LM, et al. Neuroscientist. 2005 Aug;11(4):323-33. doi: 10.1177/1073858404272255. Neuroscientist. 2005. PMID: 16061519 Review. - Modulation of multiple memory systems: from neurotransmitters to metabolic substrates.
Gold PE, Newman LA, Scavuzzo CJ, Korol DL. Gold PE, et al. Hippocampus. 2013 Nov;23(11):1053-65. doi: 10.1002/hipo.22182. Hippocampus. 2013. PMID: 23929581 Free PMC article. Review.
Cited by
- The role of physical activity in the physiological activation of the scholastic pre-requirements.
Latino F, Tafuri F. Latino F, et al. AIMS Neurosci. 2024 Aug 19;11(3):244-259. doi: 10.3934/Neuroscience.2024016. eCollection 2024. AIMS Neurosci. 2024. PMID: 39431273 Free PMC article. - Sedentary behavior, brain-derived neurotrophic factor and brain structure in midlife: A longitudinal brain MRI sub-study of the coronary artery risk development in young adults study.
Zhang X, Meirelles OD, Li Z, Yaffe K, Bryan RN, Qiu C, Launer LJ. Zhang X, et al. Front Dement. 2023 Mar 13;2:1110553. doi: 10.3389/frdem.2023.1110553. eCollection 2023. Front Dement. 2023. PMID: 39081995 Free PMC article. - Specific exercise patterns generate an epigenetic molecular memory window that drives long-term memory formation and identifies ACVR1C as a bidirectional regulator of memory in mice.
Keiser AA, Dong TN, Kramár EA, Butler CW, Chen S, Matheos DP, Rounds JS, Rodriguez A, Beardwood JH, Augustynski AS, Al-Shammari A, Alaghband Y, Alizo Vera V, Berchtold NC, Shanur S, Baldi P, Cotman CW, Wood MA. Keiser AA, et al. Nat Commun. 2024 May 7;15(1):3836. doi: 10.1038/s41467-024-47996-w. Nat Commun. 2024. PMID: 38714691 Free PMC article. - Mechanisms underlying the effect of voluntary running on adult hippocampal neurogenesis.
Gao Y, Syed M, Zhao X. Gao Y, et al. Hippocampus. 2023 Apr;33(4):373-390. doi: 10.1002/hipo.23520. Epub 2023 Mar 9. Hippocampus. 2023. PMID: 36892196 Free PMC article. Review. - Hippocampus-sensitive and striatum-sensitive learning one month after morphine or cocaine exposure in male rats.
Gardner RS, Korol DL, Gold PE. Gardner RS, et al. Pharmacol Biochem Behav. 2022 Jun;217:173392. doi: 10.1016/j.pbb.2022.173392. Epub 2022 May 2. Pharmacol Biochem Behav. 2022. PMID: 35513118 Free PMC article.
References
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. 2004. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett 1:43–48. - PubMed
- Albeck DS, Sano K, Prewitt GE, Dalton L. 2006. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res 168:345–348. - PubMed
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, Rowe JW. 1995. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging 10:578–589. - PubMed
- Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. 2002. BDNF–triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus 12: 551–560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous