Receptor tyrosine kinase-mediated angiogenesis - PubMed (original) (raw)
Review
Receptor tyrosine kinase-mediated angiogenesis
Michael Jeltsch et al. Cold Spring Harb Perspect Biol. 2013.
Abstract
The endothelial cell is the essential cell type forming the inner layer of the vasculature. Two families of receptor tyrosine kinases (RTKs) are almost completely endothelial cell specific: the vascular endothelial growth factor (VEGF) receptors (VEGFR1-3) and the Tie receptors (Tie1 and Tie2). Both are key players governing the generation of blood and lymphatic vessels during embryonic development. Because the growth of new blood and lymphatic vessels (or the lack thereof) is a central element in many diseases, the VEGF and the Tie receptors provide attractive therapeutic targets in various diseases. Indeed, several drugs directed to these RTK signaling pathways are already on the market, whereas many are in clinical trials. Here we review the VEGFR and Tie families, their involvement in developmental and pathological angiogenesis, and the different possibilities for targeting them to either block or enhance angiogenesis and lymphangiogenesis.
Figures
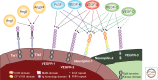
Figure 1.
Schematic presentation of Tie and VEGF receptors and their ligands. There are five VEGFs and three angiopoietins in mammals (the mouse ortholog of Ang4 is also referred to as Ang3). Dotted lines indicate that the ligand–receptor interaction is weak or nonexistent for some isoforms of the ligand (Joukov et al. 1997; Baldwin et al. 2001; Leppanen et al. 2011). CUB, Clr/Cls, urchin EGF-like protein, and bone morphogenetic protein I; CF, coagulation factor; MAM, meprin/A5-protein/PTPμ; Ig, immunoglobulin; EGF, epidermal growth factor; FN, fibronectin.
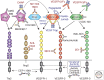
Figure 2.
Schematic presentation of the involvement of RTKs in sprouting angiogenesis. VEGF-A and VEGF-C activate VEGFR-2 and VEGFR-3 in the tip cells of angiogenic sprouts, which leads to the migratory cell phenotype. Dll4, which is expressed in the tip cells, interacts with Notch on stalk cells to down-regulate VEGFR-2 and VEGFR-3 and to up-regulate VEGFR-1. Tip cells express Ang2, and may release Ang2 from the Weibel-Palade bodies promoting angiogenesis. Pericyte-produced and matrix-associated Ang1 stabilizes the stalk cells via cell–cell junctional Tie receptor complexes, promoting cell survival, matrix interactions, and endothelial barrier function. In the stalk cells, Ang2 may compete with Ang1, promoting vessel destabilization.
Figure 3.
Schematic presentation of inhibitors of the VEGF and Tie pathways. Inhibitors approved by the FDA for clinical use are indicated in blue. Inhibitors that are currently tested in clinical trials are indicated in black and preclinical and nonclinical inhibitors in red.
Similar articles
- Vascular endothelial growth factor activates the Tie family of receptor tyrosine kinases.
Singh H, Milner CS, Aguilar Hernandez MM, Patel N, Brindle NP. Singh H, et al. Cell Signal. 2009 Aug;21(8):1346-50. doi: 10.1016/j.cellsig.2009.04.002. Epub 2009 Apr 17. Cell Signal. 2009. PMID: 19376222 - Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis.
Karkkainen MJ, Petrova TV. Karkkainen MJ, et al. Oncogene. 2000 Nov 20;19(49):5598-605. doi: 10.1038/sj.onc.1203855. Oncogene. 2000. PMID: 11114740 Review. - Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials.
Rahimi N. Rahimi N. Exp Eye Res. 2006 Nov;83(5):1005-16. doi: 10.1016/j.exer.2006.03.019. Epub 2006 May 19. Exp Eye Res. 2006. PMID: 16713597 Free PMC article. Review. - Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis.
Thurston G. Thurston G. Cell Tissue Res. 2003 Oct;314(1):61-8. doi: 10.1007/s00441-003-0749-6. Epub 2003 Aug 12. Cell Tissue Res. 2003. PMID: 12915980 Review. - Vorolanib, sunitinib, and axitinib: A comparative study of vascular endothelial growth factor receptor inhibitors and their anti-angiogenic effects.
Bakri SJ, Lynch J, Howard-Sparks M, Saint-Juste S, Saim S. Bakri SJ, et al. PLoS One. 2024 Jun 4;19(6):e0304782. doi: 10.1371/journal.pone.0304782. eCollection 2024. PLoS One. 2024. PMID: 38833447 Free PMC article.
Cited by
- Intertwined regulation of angiogenesis and immunity by myeloid cells.
Rivera LB, Bergers G. Rivera LB, et al. Trends Immunol. 2015 Apr;36(4):240-9. doi: 10.1016/j.it.2015.02.005. Epub 2015 Mar 11. Trends Immunol. 2015. PMID: 25770923 Free PMC article. Review. - Ferulic Acid Prevents Angiogenesis Through Cyclooxygenase-2 and Vascular Endothelial Growth Factor in the Chick Embryo Chorioallantoic Membrane Model.
Ekowati J, Hamid IS, Diyah NW, Siswandono S. Ekowati J, et al. Turk J Pharm Sci. 2020 Aug;17(4):424-431. doi: 10.4274/tjps.galenos.2019.44712. Epub 2020 Aug 28. Turk J Pharm Sci. 2020. PMID: 32939139 Free PMC article. - Targeting the Ang2/Tie2 Axis with Tanshinone IIA Elicits Vascular Normalization in Ischemic Injury and Colon Cancer.
Zou W, Qian C, Zhang S, Wan X, Wei Z, Li X, Wu Y, Chen W, Wang A, Zhao Y, Lu Y. Zou W, et al. Oxid Med Cell Longev. 2021 Nov 10;2021:7037786. doi: 10.1155/2021/7037786. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34804370 Free PMC article. - Tipping the balance from angiogenesis to fibrosis in CKD.
Ballermann BJ, Obeidat M. Ballermann BJ, et al. Kidney Int Suppl (2011). 2014 Nov;4(1):45-52. doi: 10.1038/kisup.2014.9. Kidney Int Suppl (2011). 2014. PMID: 26312149 Free PMC article. Review. - HUVECs-derived exosomes increase neovascularization and decrease limb necrosis in hindlimb ischemia.
Ismail MT, Anggrahini DW, Haryana SM, Setianto BY. Ismail MT, et al. Narra J. 2024 Dec;4(3):e1358. doi: 10.52225/narra.v4i3.1358. Epub 2024 Nov 22. Narra J. 2024. PMID: 39816111 Free PMC article.
References
- Abdullah SE, Perez-Soler R 2011. Mechanisms of resistance to vascular endothelial growth factor blockade. Cancer 118: 3455–3467 - PubMed
- Achen MG, Stacker SA 2008. Molecular control of lymphatic metastasis. Ann NY Acad Sci 1131: 225–234 - PubMed
- Alders M, Hogan BM, Gjini E, Salehi F, Al-Gazali L, Hennekam EA, Holmberg EE, Mannens MM, Mulder MF, Offerhaus GJ, et al. 2009. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet 41: 1272–1274 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous