Differentiation between low- and high-efficacy CB1 receptor agonists using a drug discrimination protocol for rats - PubMed (original) (raw)
Differentiation between low- and high-efficacy CB1 receptor agonists using a drug discrimination protocol for rats
Torbjörn U C Järbe et al. Psychopharmacology (Berl). 2014 Feb.
Abstract
Rationale: The "subjective high" from marijuana ingestion is likely due to Δ(9)-tetrahydrocannabinol (THC) activating the central cannabinoid receptor type 1 (CB1R) of the endocannabinoid signaling system. THC is a weak partial agonist according to in vitro assays, yet THC mimics the behavioral effects induced by more efficacious cannabinergics. This distinction may be important for understanding similarities and differences in the dose-effect spectra produced by marijuana/THC and designer cannabimimetics ("synthetic marijuana").
Objective: We evaluated if drug discrimination is able to functionally detect/differentiate between a full, high-efficacy CB1R agonist [(±)AM5983] and the low-efficacy agonist THC in vivo.
Materials and methods: Rats were trained to discriminate between four different doses of AM5983 (0.10 to 0.56 mg/kg), and vehicle and dose generalization curves were determined for both ligands at all four training doses of AM5983. The high-efficacy WIN55,212-2 and the lower-efficacy (R)-(+)-methanandamide were examined at some AM5983 training conditions. Antagonism tests involved rimonabant and WIN55,212-2 and AM5983. The separate (S)- and (R)-isomers of (±)AM5983 were tested at one AM5983 training dose (0.30 mg/kg). The in vitro cyclic adenosine monophosphate (cAMP) assay examined AM5983 and the known CB1R agonist CP55,940.
Results: Dose generalization ed50 values increased as a function of the training dose of AM5983, but more so for the partial agonists. The order of potency was (R)-isomer > (±)AM5983 > (S)-isomer and AM5983 > WIN55,212-2 ≥ THC > (R)-(+)-methanandamide. Surmountable antagonism of AM5983 and WIN55,212-2 occurred with rimonabant. The cAMP assay confirmed the cannabinergic nature of AM5983 and CP55,940.
Conclusions: Drug discrimination using different training doses of a high-efficacy, full CB1R agonist differentiated between low- and high-efficacy CB1R agonists.
Conflict of interest statement
Conflict of interest All authors declare that there is no actual or potential conflict of interest related to this manuscript. Authors declare that the study sponsor did not have any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Figures
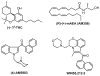
Fig. 1
Chemical structures of cannabinergics used in the drug discrimination studies
Fig. 2
Effects of the cannabinergics (±)-AM5983 and (−)-CP55,940 on cAMP levels in HEK-293 cells expressing rat CB1R. Cells were stimulated in the presence of 2 μM forskolin (FSK) for 30 min. The data are the average of five experiments performed in triplicates for each ligand
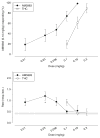
Fig. 3
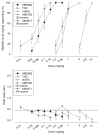
Generalization test data for AM5983 and Δ9-THC (top) and the corresponding response rate data (bottom) for rats trained to discriminate between vehicle and 0.10 mg/kg AM5983 (group 1a). Data points are based on one to two observations for each rat (n =12–18, AM5983, and n =11–22, Δ9-THC) and were obtained on separate test days. Vehicle response rate (mean ± SEM) was 0.42±0.05 responses per s (horizontal lines), based on the initial six reinforcement cycles of the nondrug maintenance sessions immediately preceding the above tests. Asterisk indicates a significant difference from the vehicle rate at P ≤0.05 (Holm–Sidak post hoc multiple comparison procedure involving a control mean)
Fig. 4
Generalization test data for four cannabinergics (top) and the corresponding response rate data (bottom) for rats trained to discriminate between vehicle and 0.18 mg/kg AM5983 (group 1b). Data points are based on one observation for each rat (n =11–12, AM5983, and n =9–12, Δ9-THC; mAEA; and WIN55,212-2) and were obtained on separate test days. Vehicle response rate (mean ± SEM) was 0.61±0.04 responses per s (horizontal lines), based on the initial six reinforcement cycles of the nondrug maintenance sessions immediately preceding the above tests. Asterisk indicates significant difference from the vehicle rate at P ≤0.05 (Holm–Sidak post hoc multiple comparison procedure involving a control mean)
Fig. 5
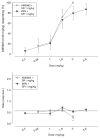
Antagonism test data for AM5983 and WIN55,212-2 (top) and the corresponding response rate data (bottom) for rats trained to discriminate between vehicle and 0.18 mg/kg AM5983 (group 1b). Tests were conducted 20 min after concurrent i.p. administrations of 1 mg/kg rimonabant (SR) and AM5983 or WIN55,212-2. Data points are based on one observation for each rat (n =8–10 for both drug combinations) and were obtained on separate test days. Vehicle response rate (mean ± SEM) was 0.50±0.03 responses per s (horizontal lines), based on the initial six reinforcement cycles of the nondrug maintenance sessions immediately preceding the above tests. Asterisk indicates a significant difference from the vehicle rate at P ≤0.05 (Holm–Sidak post hoc multiple comparison procedure involving a control mean)
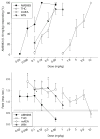
Fig. 6
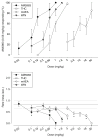
Generalization test data for five cannabinergics (top) and the corresponding response rate data (bottom) for rats trained to discriminate between vehicle and 0.30 mg/kg AM5983 (group 2a). Data points are based on one observation for each rat (n =11–12, AM5983 and AM4971; n =9–11, Δ9-THC; n =10–12, mAEA; and n =10–11, AM5760) and were obtained on separate test days. Vehicle response rate (mean ± SEM) was 0.46±0.03 responses per s (horizontal lines), based on the initial six reinforcement cycles of the nondrug maintenance sessions immediately preceding the above tests. Asterisk indicates a significant difference from the vehicle rate at P ≤0.05 (Holm–Sidak post hoc multiple comparison procedure involving a control mean)
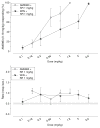
Fig. 7
Generalization test data for four cannabinergics (top) and the corresponding response rate data (bottom) for rats trained to discriminate between vehicle and 0.56 mg/kg AM5983 (group 2b). Data points are based on one observation for each rat (n =8–12, AM5983; n =8–9, Δ9-THC; n =8–10, mAEA; and n =8–9, WIN55,212-2) and were obtained on separate test days. Vehicle response rate (mean ± SEM) was 0.84±0.18 responses per s (horizontal lines), based on the initial six reinforcement cycles of the nondrug maintenance sessions immediately preceding the above tests. Asterisk indicates a significant difference from the vehicle rate at P ≤0.05 (Holm–Sidak post hoc multiple comparison procedure involving a control mean)
Fig. 8
Antagonism test data for AM5983 and WIN55,212-2 (top) and the corresponding response rate data (bottom) for rats trained to discriminate between vehicle and 0.56 mg/kg AM5983 (group 2b). Tests were conducted 20 min after concurrent i.p. administrations of 1 mg/kg rimonabant (SR) and AM5983 or WIN55,212-2. Data points are based on one observation for each rat (n =8–9 for both drug combinations) and were obtained on separate test days. Vehicle response rate (mean ± SEM) was 0.57±0.04 responses per s (horizontal lines), based on the initial six reinforcement cycles of the nondrug maintenance sessions immediately preceding the above tests. Asterisk indicates a significant difference from the vehicle rate at P ≤0.05 (Holm–Sidak post hoc multiple comparison procedure involving a control mean)
Fig. 9
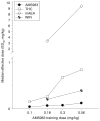
Graphical display of the changes in milligram per kilogram of the ED50 values (_Y_-axis) for the four study cannabinergics as a function of the training dose (range, 0.10 to 0.56 mg/kg) of AM5983 (_X_-axis)
Similar articles
- Central mediation and differential blockade by cannabinergics of the discriminative stimulus effects of the cannabinoid CB1 receptor antagonist rimonabant in rats.
Järbe TU, LeMay BJ, Vemuri VK, Vadivel SK, Zvonok A, Makriyannis A. Järbe TU, et al. Psychopharmacology (Berl). 2011 Aug;216(3):355-65. doi: 10.1007/s00213-011-2226-3. Epub 2011 Mar 3. Psychopharmacology (Berl). 2011. PMID: 21369753 Free PMC article. - Discriminative stimulus functions of methanandamide and delta(9)-THC in rats: tests with aminoalkylindoles (WIN55,212-2 and AM678) and ethanol.
Järbe TU, Li C, Vadivel SK, Makriyannis A. Järbe TU, et al. Psychopharmacology (Berl). 2010 Jan;208(1):87-98. doi: 10.1007/s00213-009-1708-z. Epub 2009 Nov 10. Psychopharmacology (Berl). 2010. PMID: 19902182 Free PMC article. - Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice.
McMahon LR, Ginsburg BC, Lamb RJ. McMahon LR, et al. Psychopharmacology (Berl). 2008 Jul;198(4):487-95. doi: 10.1007/s00213-007-0900-2. Epub 2007 Aug 3. Psychopharmacology (Berl). 2008. PMID: 17673980 Free PMC article. - [Drug discrimination properties and cytotoxicity of the cannabinoid receptor ligands].
Tomiyama K, Funada M. Tomiyama K, et al. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2012 Jun;47(3):135-43. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2012. PMID: 22894054 Review. Japanese. - Pharmacological and Toxicological Effects of Synthetic Cannabinoids and Their Metabolites.
Tai S, Fantegrossi WE. Tai S, et al. Curr Top Behav Neurosci. 2017;32:249-262. doi: 10.1007/7854_2016_60. Curr Top Behav Neurosci. 2017. PMID: 28012093 Free PMC article. Review.
Cited by
- Apparent CB1 Receptor Rimonabant Affinity Estimates: Combination with THC and Synthetic Cannabinoids in the Mouse In Vivo Triad Model.
Grim TW, Morales AJ, Thomas BF, Wiley JL, Endres GW, Negus SS, Lichtman AH. Grim TW, et al. J Pharmacol Exp Ther. 2017 Jul;362(1):210-218. doi: 10.1124/jpet.117.240192. Epub 2017 Apr 25. J Pharmacol Exp Ther. 2017. PMID: 28442584 Free PMC article. - Effects of cannabinoid agonists and antagonists in male rats discriminating the synthetic cannabinoid AM2201.
AlKhelb D, Burke EL, Zvonok A, Iliopoulos-Tsoutsouvas C, Georgiadis MO, Jiang S, Ho TC, Nikas SP, Makriyannis A, Desai RI. AlKhelb D, et al. Eur J Pharmacol. 2023 Dec 5;960:176168. doi: 10.1016/j.ejphar.2023.176168. Epub 2023 Oct 29. Eur J Pharmacol. 2023. PMID: 38059442 Free PMC article. - Molecular Mechanism and Cannabinoid Pharmacology.
Schurman LD, Lu D, Kendall DA, Howlett AC, Lichtman AH. Schurman LD, et al. Handb Exp Pharmacol. 2020;258:323-353. doi: 10.1007/164_2019_298. Handb Exp Pharmacol. 2020. PMID: 32236882 Free PMC article. - Human Drug Discrimination: Elucidating the Neuropharmacology of Commonly Abused Illicit Drugs.
Bolin BL, Alcorn JL, Reynolds AR, Lile JA, Stoops WW, Rush CR. Bolin BL, et al. Curr Top Behav Neurosci. 2018;39:261-295. doi: 10.1007/7854_2016_10. Curr Top Behav Neurosci. 2018. PMID: 27272070 Free PMC article. Review. - Cross-substitution of Δ9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats.
Wiley JL, Lefever TW, Cortes RA, Marusich JA. Wiley JL, et al. Pharmacol Biochem Behav. 2014 Sep;124:123-8. doi: 10.1016/j.pbb.2014.05.016. Epub 2014 Jun 2. Pharmacol Biochem Behav. 2014. PMID: 24887450 Free PMC article.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-Methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. - PubMed
- Adams IB, Compton DR, Martin BR. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther. 1998;284:1209–1217. - PubMed
- Alici T, Appel JB. Increasing the selectivity of the discriminative stimulus effects of delta-9-tetrahydrocannabinol: complete substitution with methanandamide. Pharmacol Biochem Behav. 2004;79:431–437. - PubMed
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN. Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAAergic ligands. Psychopharmacol (Berl) 2000;153:67–84. - PubMed
- Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol. 2010;80:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5R01 DA 007215-18/DA/NIDA NIH HHS/United States
- 5R01 DA 009064-19/DA/NIDA NIH HHS/United States
- R01 DA007215/DA/NIDA NIH HHS/United States
- P01 DA009158/DA/NIDA NIH HHS/United States
- R01 DA009064/DA/NIDA NIH HHS/United States
- R37 DA023142/DA/NIDA NIH HHS/United States
- 5R3 7DA 023142-05/DA/NIDA NIH HHS/United States
- 5P01 DA 009158-14/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources