Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma - PubMed (original) (raw)
Comparative Study
Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma
Risheng Que et al. World J Surg Oncol. 2013.
Abstract
Background: Altered expression of serum microRNAs (miRNAs) have been reported to correlate with carcinogenesis and progression of pancreatic adenocarcinoma (PC), but descriptions of serum exosomal miRNAs in PC are still lacking. This study was designed to evaluate serum exosomal miRNA levels in PC patients and to investigate their relationships with clinicopathologic features and prognosis.
Methods: Four miRNAs (miR-17-5p, miR-21, miR-155 and miR-196a) related to PC were selected for examination in our research. Serum miRNA was examined by RT-PCR in a group of 49 patients, including 22 with PCs, 6 with benign pancreatic tumors, 7 with ampullary carcinomas, 6 with chronic pancreatitis and 8 healthy participants. The clinicopathologic data were also collected, and PC patients were classified according to the presence of metastasis, tumor differentiation and advanced stage.
Results: There were low expressions of exosomal miR-155 and miR-196a in serum samples of PC patients when U-6 was used as a control. Serum exosomal miR-17-5p was higher in PC patients than in non-PC patients and healthy participants. High levels of miR-17-5p were significantly correlated with metastasis and advanced stage of PC. The serum exosomal miR-21 level in PC was higher than that in the normal and chronic pancreatitis groups, but was not significantly correlated with PC differentiation and tumor stage.
Conclusions: There were high expressions of serum exosomal miR-17-5p and miR-21 in PC patients. Examination of serum exosomal microRNA is a useful serum biomarker for PC diagnosis other than serum-free microRNA. It is postulated that exosomal miR-17-5p participates in the progression of PC.
Figures
Figure 1
Reverse transcription polymerase chain reaction amplification curves of microRNAs in serum of patients with pancreatic adenocarcinoma. U6 (A), miR-17-5p (B) and miR-21 (C) were stably expressed with mean cycle threshold values of 29.7, 29.4 and 26.6, respectively. miR-155 (D) and miR-196a (E) were found to have low expression levels.
Figure 2
Reverse transcription polymerase chain reaction dissociation curves of microRNAs in sera of patients with pancreatic adenocarcinoma. Circulating U6 (A), miR-17-5p (B) and miR-21 (C) miRNAs were specifically amplified with a singular peak in the dissociation curve. The variation in the dissociation curves of miR-155 (D) and miR-196a (E) indicated negative expression.
Figure 3
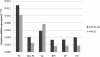
Relative expression levels of circulating miR-17-5p and miR-21. miR-17-5p and miR-21 levels were significantly higher in patients with PC than in healthy participants (HPs) and the non–primary carcinoma (non-PC) group, with 3.16- and 4.05-fold changes compared with the non-PC group and 3.2- and 5.86-fold changes compared with HPs, respectively.
Figure 4
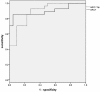
Receiver operator characteristic curves of miR-17-5p and miR-21 for discriminating non-PC from PC. Receiver operating characteristic areas under the curve of miR-17-5p and miR-21 were 0.887 (95% confidence interval (CI) = 0.796 to 0.978) and 0.897 (95% CI = 0.803 to 0.991), respectively. At the cutoff values of 6.826 for miR-17-5p and 7.693 for miR-21, specificities and sensitivities were 72.7% and 92.6% for miR-17-5p and 95.5% and 81.5% for miR-21, respectively.
Figure 5
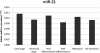
Relative expression level of circulating miR-21 in each group. On the basis of RT-PCR assay of miR-21, there were 1.23-, 1.28- and 1.13-fold changes between the different stage, differentiation and metastasis groups of PC patients (P = 0.339, 0.385 and 0.668, respectively), indicating no significant correlations with stage, differentiation or metastasis.
Figure 6
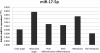
Relative expression level of circulating miR-17-5p in each group. Circulating miR-17-5p was significantly higher in the advanced stage and metastasis groups, with 2.13-fold (P = 0.01) and 2.46-fold (P = 0.017) changes, respectively. No significant difference was found between the poorly differentiated and well-differentiated groups.
Similar articles
- Exosomal onco-miRs from serum of patients with adenocarcinoma of the esophagus: comparison of miRNA profiles of exosomes and matching tumor.
Warnecke-Eberz U, Chon SH, Hölscher AH, Drebber U, Bollschweiler E. Warnecke-Eberz U, et al. Tumour Biol. 2015 Jun;36(6):4643-53. doi: 10.1007/s13277-015-3112-0. Epub 2015 Jan 29. Tumour Biol. 2015. PMID: 25631748 - Plasma miRNAs in diagnosis and prognosis of pancreatic cancer: A miRNA expression analysis.
Zhou X, Lu Z, Wang T, Huang Z, Zhu W, Miao Y. Zhou X, et al. Gene. 2018 Oct 5;673:181-193. doi: 10.1016/j.gene.2018.06.037. Epub 2018 Jun 18. Gene. 2018. PMID: 29913239 - Role of exosomal microRNA-125b-5p in conferring the metastatic phenotype among pancreatic cancer cells with different potential of metastasis.
Wu M, Tan X, Liu P, Yang Y, Huang Y, Liu X, Meng X, Yu B, Wu Y, Jin H. Wu M, et al. Life Sci. 2020 Aug 15;255:117857. doi: 10.1016/j.lfs.2020.117857. Epub 2020 May 27. Life Sci. 2020. PMID: 32470446 - Exosomal microRNA panels as biomarkers for hematological malignancies.
Moloudizargari M, Hekmatirad S, Mofarahe ZS, Asghari MH. Moloudizargari M, et al. Curr Probl Cancer. 2021 Oct;45(5):100726. doi: 10.1016/j.currproblcancer.2021.100726. Epub 2021 Mar 5. Curr Probl Cancer. 2021. PMID: 33752898 Review. - Exosomal miRNAs in the microenvironment of pancreatic cancer.
Zou X, Huang Z, Guan C, Shi W, Gao J, Wang J, Cui Y, Wang M, Xu Y, Zhong X. Zou X, et al. Clin Chim Acta. 2023 Apr 1;544:117360. doi: 10.1016/j.cca.2023.117360. Epub 2023 Apr 20. Clin Chim Acta. 2023. PMID: 37086943 Review.
Cited by
- Serum exosomal microRNAs as predictive markers for EGFR mutations in non-small-cell lung cancer.
Xia J, Luo M, Dai L, Wang L, Wang L, Zhu J. Xia J, et al. J Clin Lab Anal. 2021 May;35(5):e23743. doi: 10.1002/jcla.23743. Epub 2021 Mar 8. J Clin Lab Anal. 2021. PMID: 33682961 Free PMC article. - miRNA and Gene Expression in Pancreatic Ductal Adenocarcinoma.
Tesfaye AA, Azmi AS, Philip PA. Tesfaye AA, et al. Am J Pathol. 2019 Jan;189(1):58-70. doi: 10.1016/j.ajpath.2018.10.005. Am J Pathol. 2019. PMID: 30558723 Free PMC article. Review. - Pancreatic Cancer Small Extracellular Vesicles (Exosomes): A Tale of Short- and Long-Distance Communication.
Waldenmaier M, Seibold T, Seufferlein T, Eiseler T. Waldenmaier M, et al. Cancers (Basel). 2021 Sep 28;13(19):4844. doi: 10.3390/cancers13194844. Cancers (Basel). 2021. PMID: 34638330 Free PMC article. Review. - Exosomes: A Promising Avenue for the Diagnosis of Breast Cancer.
Meng Y, Sun J, Wang X, Hu T, Ma Y, Kong C, Piao H, Yu T, Zhang G. Meng Y, et al. Technol Cancer Res Treat. 2019 Jan 1;18:1533033818821421. doi: 10.1177/1533033818821421. Technol Cancer Res Treat. 2019. PMID: 30760122 Free PMC article. Review. - Extracellular vesicles as shuttles of tumor biomarkers and anti-tumor drugs.
Zocco D, Ferruzzi P, Cappello F, Kuo WP, Fais S. Zocco D, et al. Front Oncol. 2014 Oct 8;4:267. doi: 10.3389/fonc.2014.00267. eCollection 2014. Front Oncol. 2014. PMID: 25340037 Free PMC article. Review.
References
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;11:2257–2261. doi: 10.1073/pnas.0510565103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical