Prediction and Prevention of Prescription Drug Abuse: Role of Preclinical Assessment of Substance Abuse Liability - PubMed (original) (raw)
Prediction and Prevention of Prescription Drug Abuse: Role of Preclinical Assessment of Substance Abuse Liability
Julie A Marusich et al. Methods Rep RTI Press. 2013 Jul.
Abstract
In 2011, the prevalence of prescription drug abuse exceeded that of any other illicit drug except marijuana. Consequently, efforts to curtail abuse of new medications should begin during the drug development process, where abuse liability can be identified and addressed before a candidate medication has widespread use. The first step in this process is scheduling with the Drug Enforcement Agency so that legal access is appropriately restricted, dependent upon levels of abuse risk and medical benefit. To facilitate scheduling, the Food and Drug Administration (FDA) has published guidance for industry that describes assessment of abuse liability. The purpose of this paper is to review methods that may be used to satisfy the FDA's regulatory requirements for animal behavioral and dependence pharmacology. Methods include psychomotor activity, self-administration (an animal model of the rewarding effects of a drug), drug discrimination (an animal model of the subjective effects of a drug), and evaluation of tolerance and dependence. Data from tests conducted at RTI with known drugs of abuse illustrate typical results, and demonstrate that RTI is capable of performing these tests. While using preclinical data to predict abuse liability is an imperfect process, it has substantial predictive validity. The ultimate goal is to increase consumer safety through appropriate scheduling of new medications.
Figures
Figure 1
Standard locomotor chamber used for measuring psychomotor behavior
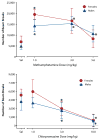
Figure 2. Male and female Sprague-Dawley rats exhibited increased locomotor activity compared to drug vehicle (saline) when injected with the psychomotor stimulant methamphetamine (top panel), as evidenced by an increase in the number of beam breaks
Rats exhibited decreased activity when injected with the prototypic antipsychotic chlorpromazine, as evidenced by fewer and fewer beam breaks as the dose of chlorpromazine increased (bottom panel). These data indicate that the procedure has sufficient sensitivity to detect both increases and decreases in motor activity. Note the different Y-axis scales. Sal stands for saline vehicle. Asterisks indicate significant differences from vehicle (p < 0.05).
Figure 3
Standard operant chamber used for drug self-administration
Figure 4. Male Sprague-Dawley rats were trained to press a lever to self-administer i.v. infusions of cocaine (five lever presses were required for each infusion)
The figure shows the average number of lever presses as a function of dose of drug across 3 days of access to each dose on the active (filled symbols) and inactive (open symbols) levers. “Sal” stands for saline vehicle. Rats pressed the active lever to receive infusions of cocaine and methamphetamine at much higher rates than for infusions of saline, indicating that these drugs are reinforcing. Responding for higher doses of cocaine and methamphetamine dropped to saline levels, whereas lower doses of cocaine and methamphetamine also resulted in lesser responding. This inverted U-shaped dose-effect function is typical of self-administration of many known drugs of abuse and may represent the incapacitating effect of higher doses and the lack of reinforcing effect from lower doses. MDPV produced effects that were similar to those of cocaine but at lower doses, suggesting that MDPV was also reinforcing to the rats and was more potent than cocaine. MDPV, a synthetic cathinone that has been commonly identified in products labeled “bath salts,” was recently designated by the DEA as a schedule I drug due to its abuse in humans. In contrast, the antipsychotic chlorpromazine was not self-administered and, in fact, shows signs that it may be aversive, in that it produced lower than baseline rates of infusions.
Figure 5
Standard operant chamber used for drug discrimination
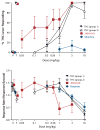
Figure 6. Two groups of male Sprague-Dawley rats were trained to discriminate 3 mg/kg THC from vehicle
For both panels, V stands for vehicle and T stands for the training dose (3 mg/kg THC). The data points above these labels show data from when animals were trained to discriminate THC from vehicle. In control tests with vehicle and 3 mg/kg THC (left side of top panel), rats in both groups responded almost exclusively on the appropriate lever. When other doses of THC were substituted for the 3 mg/kg training dose, dose-dependent substitution occurred, with rats responding most on the drug lever at doses at or above the training dose (top panel, unfilled black triangles and filled inverted black triangles). In group 1, dose-dependent substitution also occurred upon substitution of JWH-018 (top panel, filled red squares), a synthetic cannabinoid that has been abused by humans in products marketed under names such as “Spice.” Lower doses of JWH-018 substituted for THC, suggesting that JWH-018 is more potent than THC. Consistent with the high predictive validity of this model, humans who have ingested these products report a marijuana-like intoxication. In contrast, rats that were injected with morphine (group 2) did not respond on the THC-associated lever (top panel, filled blue circles), suggesting that morphine failed to substitute. As predicted by the model, morphine does not possess marijuana-like subjective effects in humans, although it does share subjective effects with other mu opioid agonists such as heroin. Together, these results demonstrate the pharmacological selectivity and predictive validity of the drug discrimination procedure. Overall response rates calculated as responses per second, a measure of nonspecific, direct effects of the drugs (e.g., effects on motor behavior), are shown in the bottom panel.
Similar articles
- Abuse liability assessment in preclinical drug development: predictivity of a translational approach for abuse liability testing using methylphenidate in four standardized preclinical study models.
Teuns GB, Geys HM, Geuens SM, Stinissen P, Meert TF. Teuns GB, et al. J Pharmacol Toxicol Methods. 2014 Nov-Dec;70(3):295-309. doi: 10.1016/j.vascn.2014.02.002. Epub 2014 Mar 12. J Pharmacol Toxicol Methods. 2014. PMID: 24632211 - Drug discrimination: A versatile tool for characterization of CNS safety pharmacology and potential for drug abuse.
Swedberg MD. Swedberg MD. J Pharmacol Toxicol Methods. 2016 Sep-Oct;81:295-305. doi: 10.1016/j.vascn.2016.05.011. Epub 2016 May 25. J Pharmacol Toxicol Methods. 2016. PMID: 27235786 Review. - Principles of assessment of abuse liability: US legal framework and regulatory environment.
Rocha BA. Rocha BA. Behav Pharmacol. 2013 Sep;24(5-6):403-9. doi: 10.1097/FBP.0b013e328363d163. Behav Pharmacol. 2013. PMID: 23820327 Review. - Narrative Review: The FDA's Perfunctory Approach of Dietary Supplement Regulations Giving Rise to Copious Reports of Adverse Events.
Li W, Wertheimer A. Li W, et al. Innov Pharm. 2023 Oct 10;14(1):10.24926/iip.v14i1.4989. doi: 10.24926/iip.v14i1.4989. eCollection 2023. Innov Pharm. 2023. PMID: 38035313 Free PMC article. Review. - Preclinical assessment of abuse liability of biologics: In defense of current regulatory control policies.
Gauvin DV, Zimmermann ZJ, Baird TJ. Gauvin DV, et al. Regul Toxicol Pharmacol. 2015 Oct;73(1):43-54. doi: 10.1016/j.yrtph.2015.06.009. Epub 2015 Jun 21. Regul Toxicol Pharmacol. 2015. PMID: 26107292 Review.
Cited by
- Cross-substitution of Δ9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats.
Wiley JL, Lefever TW, Cortes RA, Marusich JA. Wiley JL, et al. Pharmacol Biochem Behav. 2014 Sep;124:123-8. doi: 10.1016/j.pbb.2014.05.016. Epub 2014 Jun 2. Pharmacol Biochem Behav. 2014. PMID: 24887450 Free PMC article. - Baths salts, spice, and related designer drugs: the science behind the headlines.
Baumann MH, Solis E Jr, Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL. Baumann MH, et al. J Neurosci. 2014 Nov 12;34(46):15150-8. doi: 10.1523/JNEUROSCI.3223-14.2014. J Neurosci. 2014. PMID: 25392483 Free PMC article. Review. - Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat.
Wakley AA, Wiley JL, Craft RM. Wakley AA, et al. Drug Alcohol Depend. 2014 Oct 1;143:22-8. doi: 10.1016/j.drugalcdep.2014.07.029. Epub 2014 Aug 2. Drug Alcohol Depend. 2014. PMID: 25131716 Free PMC article. - Benzodiazepine and neuroactive steroid combinations in rats: anxiolytic-like and discriminative stimulus effects.
Gunter BW, Jones SA, Paul IA, Platt DM, Rowlett JK. Gunter BW, et al. Psychopharmacology (Berl). 2016 Sep;233(17):3237-47. doi: 10.1007/s00213-016-4369-8. Epub 2016 Jun 29. Psychopharmacology (Berl). 2016. PMID: 27356519 Free PMC article. - Conditioned place preference following concurrent treatment with 3, 4-methylenedioxypyrovalerone (MDPV) and methamphetamine in male and female Sprague-Dawley rats.
Risca HI, Zuarth-Gonzalez JD, Baker LE. Risca HI, et al. Pharmacol Biochem Behav. 2020 Nov;198:173032. doi: 10.1016/j.pbb.2020.173032. Epub 2020 Sep 1. Pharmacol Biochem Behav. 2020. PMID: 32888971 Free PMC article.
References
- Appel JB, Baker LE, Barrett RL, Broadbent J, Michael EM, Riddle EE, Van Groll BJ. Use of drug discrimination in drug abuse research. NIDA Research Monograph. 1991;116:369–397. - PubMed
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug and Alcohol Dependence. 2003;70:S55–S72. - PubMed
- Balster RL, Chait LD. The effects of phencyclidine on amphetamine stereotypy in rats. European Journal of Pharmacology. 1978;48:445–450. - PubMed
- Barrett RJ, Caul WF, Smith R. Withdrawal, tolerance, and sensitization to dopamine mediated interoceptive cues in rats trained on a three-lever drug-discrimination task. Pharmacology, Biochemistry, and Behavior. 2005;81:1–8. - PubMed
- Calabrese EJ. Addiction and dose response: The psychomotor stimulant theory of addiction reveals that hormetic dose responses are dominant. Critical Reviews in Toxicology. 2008;387:599–617. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources