CD4(+) T cell activation promotes the differential release of distinct populations of nanosized vesicles - PubMed (original) (raw)
CD4(+) T cell activation promotes the differential release of distinct populations of nanosized vesicles
Els J van der Vlist et al. J Extracell Vesicles. 2012.
Abstract
Many cell types release nanosized vesicles derived from endosomal compartments (exosomes) or the plasma membrane. Vesicles actively released by CD4(+) T cells have immune-modulatory characteristics. Using our recently developed high-resolution flow cytometry-based method for the analysis of individual nanosized vesicles, we here investigated how T cell receptor (TCR)-triggering and co-stimulatory signals influence the quantity and characteristics of nanosized vesicles released by CD4(+) T cells. We found that the number of released nanosized vesicles within the buoyant density range characteristic for exosomes (1.10-1.19 g/ml) was increased by TCR-triggering and that additional co-stimulatory signals had a potentiating effect on vesicle release. However, the increase in the number of released vesicles varied substantially between density fractions within the 1.10-1.19 g/ml range and was highest for the vesicle populations in 1.14 and 1.17 g/ml fractions. Heterogeneity was also observed within the individual density fractions. Based on lipid bilayer fluorescent labelling intensity and light scattering, 3 distinct vesicle subpopulations were identified. One vesicle subpopulation increased significantly more upon T cell activation than the other subpopulations, and this was dependent on high levels of co-stimulation. These data show that T cells release a heterogeneous population of nanosized vesicles and indicate that T cells differentially regulate the release of distinct vesicle subpopulations depending on their activation status.
Keywords: NTA; T cells; exosomes; extracellular vesicles; flow cytometry; immune regulation; microparticles; microvesicles; nanosized vesicles.
Figures
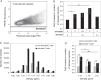
Fig. 1
Analysis of T cell activation after different levels of TCR- and co-stimulation triggering. CD4+ T cells (KO4C1) were activated by TCR-triggering (0.1 or 10 µg/ml plate-bound anti-CD3), with or without additional co-stimulation (0.5 or 5 µg/ml plate-bound anti-CD28) for 20 hours and compared to non-activated T cells. Cells were analysed for CD69 upregulation (A), TCR downregulation (B) and IFN-gamma production (C) by flow cytometry. Histograms (top panels) show the expression of indicated markers on non-activated T cells (grey line) or on T cells activated with anti-CD3 (10 µg/ml) and anti-CD28 (5 µg/ml) (black line) or isotype control stainings (filled histograms). The bar graphs (bottom panels) show the geometric means expressed as percentages of maximal expression of the indicated markers (set to 100%) of at least 3 independent experiments.
Fig. 2
Quantitative analysis of nanosized vesicles released by T cells. KO4C1 T cells were activated as described in Fig. 1. Vesicles released by T cells were isolated from culture supernatants, labelled with the fluorescent membrane dye PKH67 and floated to equilibrium density into a sucrose gradient. The vesicles in the collected sucrose gradient fractions were analysed by fluorescence-based high-resolution flow cytometry. (A) Dot plot of reduced wide-angle FSC versus PKH67 fluorescence representing fluorescent vesicles derived from non-activated T cells (from pooled fractions with densities of 1.12–1.17 g/ml). (B) Fluorescently labelled vesicles from differently activated T cells were quantified using our time-based quantification method. Indicated are the average and standard deviation of the number of vesicles released of at least 3 independent experiments (from fractions with densities of 1.10–1.19 g/ml). The number of vesicles derived from non-activated T cells was set to 1. (C) Fluorescently labelled vesicles from non-activated or activated (10 µg/ml anti-CD3 + 5 µg/ml anti-CD28) T cells were quantified using our time-based quantification method. Indicated is the number of events measured in 30 seconds in the indicated sucrose gradient density fractions. One representative out of 6 experiments is shown (D) Fold increase in the number of released vesicles upon activation (10 µg/ml anti-CD3 + 5 µg/ml anti-CD28) of T cells per density fraction. Indicated are the averages and standard deviations of 9 independent experiments. The number of vesicles released by non-activated T cells was set to 1 for each density fraction. Asterisks denote significant differences (* p<0.05, ** p≤0.01, *** p≤0.001).
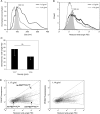
Fig. 3
Heterogeneity in T cell-derived vesicle populations with different buoyant densities. Vesicles released by non-activated T cells were isolated and analysed as described in Fig. 2. Histograms indicating size as determined by NTA (A) or reduced wide-angle FSC as determined by high-resolution flow cytometry (B) of 100 nm beads (filled histograms, light grey) and T cell-derived vesicles in fractions with densities of 1.17 g/ml (black line) or 1.14 g/ml (dark grey line). (C) Reduced wide-angle FSC geometric means of vesicles from non-activated T cells with densities of 1.17 or 1.14 g/ml. Indicated are the averages and standard deviation of geometric mean values of 6 independent experiments. Asterisks denote significant differences (**p ≤ 0.01). Histograms in (A) and (B) are from the same experiment, representative of 6 (A) or 5 (B) experiments. (D) Dot plots representing wide-angle FSC and PKH67 fluorescence of vesicles from non-activated T cells floating at 1.17 (left) and 1.14 g/ml (right). Gates were set around vesicles subpopulations with low reduced wide-angle FSC levels and low fluorescence (FSClowFLlow), high(er) reduced wide-angle FSC levels and high(er) fluorescence (FSChighFLhigh) or high(er) reduced wide-angle FSC levels and low fluorescence (FSChighFLlow).
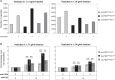
Fig. 4
T cells differentially regulate the release of distinct vesicle subpopulations upon different activation signals. Vesicles from activated (10 µg/ml anti-CD3 + 5 µg/ml anti-CD28) and non-activated T cells were isolated and analysed as described in Fig. 2. (A) Time-based quantification of T cell-derived vesicles in different density fractions in the 3 different vesicle subpopulation gates, as described in Fig. 3D. Indicated are the numbers of vesicles per gate measured in 30 seconds. One representative experiment out of 4 is shown. (B) Indicated is the average fold increase ± SD in number of vesicles released by T cells, per FSC-FL gate, activated by TCR-triggering alone or with additional co-stimulation, relative to the number of vesicles from non-activated T cells (set to 1). Averages and standard deviations are displayed for vesicles with a density of 1.17 (left graph) or 1.14 g/ml (right graph) of 4 independent experiments. Asterisks denote significant differences (** p≤0.01, *** p≤0.001).
Similar articles
- Application of high-sensitivity flow cytometry in combination with low-voltage scanning electron microscopy for characterization of nanosized objects during platelet concentrate storage.
Fedorov A, Kondratov K, Kishenko V, Mikhailovskii V, Kudryavtsev I, Belyakova M, Sidorkevich S, Vavilova T, Kostareva A, Sirotkina O, Golovkin A. Fedorov A, et al. Platelets. 2020;31(2):226-235. doi: 10.1080/09537104.2019.1599337. Epub 2019 Apr 12. Platelets. 2020. PMID: 30977703 - Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles.
Nolte-'t Hoen EN, van der Vlist EJ, Aalberts M, Mertens HC, Bosch BJ, Bartelink W, Mastrobattista E, van Gaal EV, Stoorvogel W, Arkesteijn GJ, Wauben MH. Nolte-'t Hoen EN, et al. Nanomedicine. 2012 Jul;8(5):712-20. doi: 10.1016/j.nano.2011.09.006. Epub 2011 Oct 22. Nanomedicine. 2012. PMID: 22024193 Free PMC article. - Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles.
Muhsin-Sharafaldine MR, Saunderson SC, Dunn AC, Faed JM, Kleffmann T, McLellan AD. Muhsin-Sharafaldine MR, et al. Oncotarget. 2016 Aug 30;7(35):56279-56294. doi: 10.18632/oncotarget.10783. Oncotarget. 2016. PMID: 27462921 Free PMC article. - TRP Channel Trafficking.
Planells-Cases R, Ferrer-Montiel A. Planells-Cases R, et al. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review. - Gliocrine System: Astroglia as Secretory Cells of the CNS.
Vardjan N, Parpura V, Verkhratsky A, Zorec R. Vardjan N, et al. Adv Exp Med Biol. 2019;1175:93-115. doi: 10.1007/978-981-13-9913-8_4. Adv Exp Med Biol. 2019. PMID: 31583585 Free PMC article. Review.
Cited by
- International Society for Extracellular Vesicles: first annual meeting, April 17-21, 2012: ISEV-2012.
Araldi E, Krämer-Albers EM, Hoen EN, Peinado H, Psonka-Antonczyk KM, Rao P, van Niel G, Yáñez-Mó M, Nazarenko I. Araldi E, et al. J Extracell Vesicles. 2012 Dec 28;1:19995. doi: 10.3402/jev.v1i0.19995. eCollection 2012. J Extracell Vesicles. 2012. PMID: 26082071 Free PMC article. - T Lymphocyte and CAR-T Cell-Derived Extracellular Vesicles and Their Applications in Cancer Therapy.
Calvo V, Izquierdo M. Calvo V, et al. Cells. 2022 Feb 24;11(5):790. doi: 10.3390/cells11050790. Cells. 2022. PMID: 35269412 Free PMC article. Review. - Intra- and Extracellular Effector Vesicles From Human T And NK Cells: Same-Same, but Different?
Lettau M, Janssen O. Lettau M, et al. Front Immunol. 2021 Dec 23;12:804895. doi: 10.3389/fimmu.2021.804895. eCollection 2021. Front Immunol. 2021. PMID: 35003134 Free PMC article. Review. - ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins.
Villarroya-Beltri C, Baixauli F, Mittelbrunn M, Fernández-Delgado I, Torralba D, Moreno-Gonzalo O, Baldanta S, Enrich C, Guerra S, Sánchez-Madrid F. Villarroya-Beltri C, et al. Nat Commun. 2016 Nov 24;7:13588. doi: 10.1038/ncomms13588. Nat Commun. 2016. PMID: 27882925 Free PMC article. - 2,2'4,4'-Tetrabromodiphenyl Ether (PBDE-47) Modulates the Intracellular miRNA Profile, sEV Biogenesis and Their miRNA Cargo Exacerbating the LPS-Induced Pro-Inflammatory Response in THP-1 Macrophages.
Longo V, Longo A, Adamo G, Fiannaca A, Picciotto S, La Paglia L, Romancino D, La Rosa M, Urso A, Cibella F, Bongiovanni A, Colombo P. Longo V, et al. Front Immunol. 2021 May 7;12:664534. doi: 10.3389/fimmu.2021.664534. eCollection 2021. Front Immunol. 2021. PMID: 34025666 Free PMC article.
References
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. - PubMed
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature Rev. 2009;9:581–93. - PubMed
- Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–41. - PubMed
- Martinez-Lorenzo MJ, Anel A, Gamen S, Monle nI, Lasierra P, Larrad L, et al. Activated human T cells release bioactive fas ligand and APO2 ligand in microvesicles. J Immunol. 1999;163:1274–81. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials