Thrombin generation assay and transmission electron microscopy: a useful combination to study tissue factor-bearing microvesicles - PubMed (original) (raw)
Thrombin generation assay and transmission electron microscopy: a useful combination to study tissue factor-bearing microvesicles
Damien Gheldof et al. J Extracell Vesicles. 2013.
Abstract
Introduction: Patients with cancer have a 7- to 10-fold increased risk of developing venous thromboembolism. Circulating microvesicles could be a useful predictive biomarker for venous thromboembolism in cancer. Validated and standardised techniques that could be used to determine the complete microvesicle phenotype are required.
Objectives: These were two-fold: a) to characterise tissue factor (TF)-bearing microvesicles released by cultured breast cancer cells MDA-MB-231 by flow cytometry (FCM), transmission electron microscopy (TEM) and thrombin generation assay (TGA); and b) to validate the sensitivity and variability intra/inter-assay of TGA as a useful method to study the procoagulant activity (PCA) of microvesicles.
Methods: Cultured breast cancer cells MDA-MB-231 were incubated for 45 minutes at 37°C. Samples were then centrifuged or not at 4,500 g for 15 minutes, and cells and MVs or MV-containing supernatants were used for TEM, FCM and TGA. In activity assays, microvesicles (i.e. cell-depleted supernatants) were incubated with anti-TF antibodies or with annexin V to assess the contribution of TF and phospholipids to the PCA. Alternatively, supernatants were filtered through 0.1, 0.22, 0.45 or 0.65 µm membranes and subjected to TGA.
Results: The majority of the PCA was associated with microvesicles smaller than 0.1 µm, and the mean microvesicle size estimated by TEM after 10,000 g centrifugation was 121±54 nm with a majority of vesicles between 100 and 200 nm. Microvesicles derived from 5,000 MDA-MB-231cells/ml were sufficient to significantly increase the thrombin generation of normal pooled plasma.
Conclusions: TEM, FCM and filtration coupled to TGA represent a useful combination to study the PCA of TF-bearing microvesicles, whatever their size. And it will be interesting to implement these techniques in patients.
Keywords: cancer; microvesicles; thrombin generation; tissue factor.
Figures
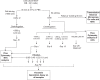
Fig. 1
Sample preparation. All supernatants were freshly used for TEM, FCM and TGA. PBS: Phosphate Buffer Saline. Sup: Supernatant. Filt: Filtrate. UC: ultracentrifugation.
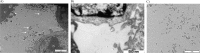
Fig. 2
Transmission electron microscopy pictures of cells with derived MVs (white arrows) after ultracentrifugation at 10,000 g during 90 minutes. A) Scale bar=2 µm. N: nucleus C: cytoplasm; B) scale bar=500 nm; C) scale bar=2 µm.
Fig. 3
Expression of TF (CD142) and MUC-1 (CD227) on MVs depleted of cells. A) Tumour microparticle analysis. Dual fluorescence analysis of MDA-MB-231 MVs stained with CD227 fluorescein isothiocyanate (FITC) (FL1) and CD142– phycoerythrin (PE) (FL2). CD142+ CD227+ MVs are represented as green dots, CD142+ CD227– MVs as red dots, CD227+ CD142− MVs as blue dots and background noise or other MVs as orange dots. Percentage and absolute number (/µl) of each subpopulation are indicated; B) backgating of CD142+ CD227+ MVs (green dots), CD142+ CD227– MVs (red dots) and CD227+ CD142– MVs (blue dots) on FSC log-SSC log cytogram; C) expression of TF (CD142) and MUC-1 (CD227) on PBS without MVs; D) FSC-SSC of control PBS without MVs labelled similarly with CD142 and CD227. E) FSC-SSC dot plot of 500 and 900 nm beads used to gate on MVs.
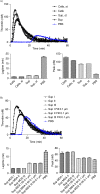
Fig. 4
Curves of thrombin generation experiments and histograms of lagtime and peak parameters. In control condition, NPP is spiked with PBS. Curve data are presented as means of n=3. All the results are representative of 3 independent experiments. A) NPP spiked with MDA-MB-231 cells (Cells) or supernatant from MDA-MB-231 cells (Sup). The supernatant shows only slightly reduced PCA as compared to cell+supernatant; B) NPP spiked with supernatant from MDA-MB-231 cells (Sup), filtered or not through membranes with various sizes (Sup Filt 0.1 µm/0.22 µm/0.45 µm/0.65 µm). Filtration through 0.65 or 0.45 µm reduces only slightly the PCA, whereas filtration through 0.22 and 0.1 µm leads to stronger reduction in PCA; C) procoagulant effect of MVs (Sup) pre-incubated with or without HTF-1 (TF-blocking Ab), TF9-10H10 (TF-non-blocking Ab) or isotypic control antibodies at 10 µg/ml. HTF-1 strongly inhibits PCA of MV-containing supernatant; D) procoagulant effect of MVs (Sup) pre-incubated with or without annexin V at 0.5 µM. Annexin V abolishes the PCA activity of the MV-containing supernatant; E) procoagulant effect of MVs derived from 100,000 cells (Sup), of the pellet obtained after ultracentrifugation of MVs (pellet UC Sup) and of the supernatant obtained after ultracentrifugation of MVs (Sup UC).
Fig. 5
Effect of stirring during cell incubation and of cell centrifugation protocol on thrombin generation. A) Thrombin generation curves and histograms of lagtime and peak parameters of normal pool plasma (PBS) spiked with MDA-MB-231 cells stirred (Cells, st) or not (Cells) or with their respective supernatant (Sup, st and Sup). No difference in PCA is observed between the stirred and non-stirred samples; B) Thrombin generation curves and histograms of lagtime and peak parameters of normal pool plasma spiked with supernatant of cells filtered or not (Sup vs. Sup Filt 0.1 µm) and produced by 3 different centrifugation protocols (
Sup I, II, III
). In control wells, NPP is spiked with PBS. The curve is representative of 3 independent experiments.
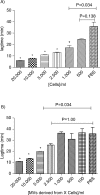
Fig. 6
Procoagulant effect of cells (Cells, A) and MVs (Sup, B) derived from different MDA-MB-231 cell concentrations (0; 100; 500; 1,000; 2,500; 5,000; 10,000; and 20,000 cells/ml) on lagtime. In control wells, NPP is spiked with PBS. Results are presented as mean±SD. Data with significant difference in comparison to control are indicated with *(p<0.05).
Similar articles
- Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation.
Nielsen T, Kristensen AF, Pedersen S, Christiansen G, Kristensen SR. Nielsen T, et al. J Extracell Vesicles. 2018 Mar 26;7(1):1454777. doi: 10.1080/20013078.2018.1454777. eCollection 2018. J Extracell Vesicles. 2018. PMID: 29696077 Free PMC article. - Inhibition of tissue factor pathway inhibitor increases the sensitivity of thrombin generation assay to procoagulant microvesicles.
Gheldof D, Mullier F, Chatelain B, Dogné JM, Chatelain C. Gheldof D, et al. Blood Coagul Fibrinolysis. 2013 Jul;24(5):567-72. doi: 10.1097/MBC.0b013e328360a56e. Blood Coagul Fibrinolysis. 2013. PMID: 23807485 - Microparticle bearing tissue factor: a link between promyelocytic cells and hypercoagulable state.
Gheldof D, Mullier F, Bailly N, Devalet B, Dogné JM, Chatelain B, Chatelain C. Gheldof D, et al. Thromb Res. 2014 Mar;133(3):433-9. doi: 10.1016/j.thromres.2013.11.008. Epub 2013 Nov 16. Thromb Res. 2014. PMID: 24290525 - The effect of corn trypsin inhibitor, anti-tissue factor pathway inhibitor antibodies and phospholipids on microvesicle-associated thrombin generation in patients with pancreatic cancer and healthy controls.
Hellum M, Franco-Lie I, Øvstebø R, Hauge T, Henriksson CE. Hellum M, et al. PLoS One. 2017 Sep 14;12(9):e0184579. doi: 10.1371/journal.pone.0184579. eCollection 2017. PLoS One. 2017. PMID: 28910348 Free PMC article. - Microvesicles as risk markers for venous thrombosis.
Rautou PE, Mackman N. Rautou PE, et al. Expert Rev Hematol. 2013 Feb;6(1):91-101. doi: 10.1586/ehm.12.74. Expert Rev Hematol. 2013. PMID: 23373784 Review.
Cited by
- The central role of extracellular vesicles in the mechanisms of thrombosis in paroxysmal nocturnal haemoglobinuria: a review.
Devalet B, Mullier F, Chatelain B, Dogné JM, Chatelain C. Devalet B, et al. J Extracell Vesicles. 2014 Mar 24;3. doi: 10.3402/jev.v3.23304. eCollection 2014. J Extracell Vesicles. 2014. PMID: 24672668 Free PMC article. Review. - Procoagulant activity of extracellular vesicles as a potential biomarker for risk of thrombosis and DIC in patients with acute leukaemia.
Gheldof D, Haguet H, Dogné JM, Bouvy C, Graux C, George F, Sonet A, Chatelain C, Chatelain B, Mullier F. Gheldof D, et al. J Thromb Thrombolysis. 2017 Feb;43(2):224-232. doi: 10.1007/s11239-016-1471-z. J Thromb Thrombolysis. 2017. PMID: 28074413 - Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin.
Tripisciano C, Weiss R, Eichhorn T, Spittler A, Heuser T, Fischer MB, Weber V. Tripisciano C, et al. Sci Rep. 2017 Jul 26;7(1):6522. doi: 10.1038/s41598-017-03262-2. Sci Rep. 2017. PMID: 28747771 Free PMC article. - Characterisation of tissue factor-bearing extracellular vesicles with AFM: comparison of air-tapping-mode AFM and liquid Peak Force AFM.
Hardij J, Cecchet F, Berquand A, Gheldof D, Chatelain C, Mullier F, Chatelain B, Dogné JM. Hardij J, et al. J Extracell Vesicles. 2013 Aug 27;2. doi: 10.3402/jev.v2i0.21045. eCollection 2013. J Extracell Vesicles. 2013. PMID: 24223257 Free PMC article. - Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation.
Nielsen T, Kristensen AF, Pedersen S, Christiansen G, Kristensen SR. Nielsen T, et al. J Extracell Vesicles. 2018 Mar 26;7(1):1454777. doi: 10.1080/20013078.2018.1454777. eCollection 2018. J Extracell Vesicles. 2018. PMID: 29696077 Free PMC article.
References
- Gerotziafas GT, Galea V, Mbemba E, Kartechi A, Sassi M, Baccouche H, et al. Tissue factor over-expression by human pancreatic cancer cells BXPC3 is related to higher prothrombotic potential as compared to breast cancer cells MCF7. Thromb Res. 2011;129:779–86. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous