Comparative marker analysis of extracellular vesicles in different human cancer types - PubMed (original) (raw)
Comparative marker analysis of extracellular vesicles in different human cancer types
Yusuke Yoshioka et al. J Extracell Vesicles. 2013.
Abstract
Several cell types, including tumour cells, secrete extracellular vesicles (EVs), and tumour-derived EVs play a role in cancer initiation and progression. These vesicles include both a common set of membrane and cytosolic proteins and origin-specific subsets of proteins that likely correlated to cell type-associated functions. To confirm the presence of EVs in the preparations, researchers have identified so-called EV marker proteins, including the tetraspanin family proteins and such cytosolic proteins as heat shock 70 kDa protein 4 (HSP70) and tumour susceptibility gene 101 (TSG101). However, studies have shown that some EV markers are not always present in all EVs, which not only complicates the identification of EVs but also precludes the quantitative evaluation of EV proteins. Thus, it is strongly required to explore well-conserved EV marker proteins that are present at similar levels, regardless of their tissue or cellular origin. In this study, we compared the presence of 11 well-known EV marker proteins by immunoblotting using EVs isolated from 4 human prostate cell lines and 5 human breast cell lines, including cancer cells with different phenotypes. We found that all the tested EVs were positive for CD9 and CD81, with similar abundance that was irrespective of the EV origin. In contrast, other EV marker proteins, such as TSG101, Rab-5b and CD63, were detected in an inconsistent manner, depending on the origin of the EVs. Thus, we propose that the detection of CD9 and/or CD81 should ensure the presence of EVs.
Keywords: CD81; CD9; breast cancer cells; extracellular vesicle marker; prostate cancer cells.
Figures
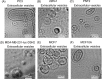
Fig. 1
Morphology of purified EVs from 6 cell lines, including cancer cells and non-cancer cells. Shown are representative phase-contrast transmission electron microscopy images of EVs from (A) PC3 cells, (B) 22Rv1 cells, (C) PNT2 cells, (D) MDA-MB-231-luc-D3H2LN cells, (E) MCF7 cells and (F) MCF10A cells. The EVs were purified from the culture supernatants of these cells using a conventional centrifugation method. The scale bar indicates 100 nm.
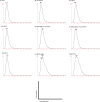
Fig. 2
Analysis of the size distribution in the EVs derived from each cell line by the NanoSight particle tracking system. (A) PC3 cells, (B) PC-3M-luc cells, (C) 22Rv1 cells, (D) PNT2 cells, (E) MDA-MB-231-luc-D3H1 cells, (F) MDA-MB-231-luc-D3H2LN cells, (G) MCF7 cells, (H) MCF7-ADR cells and (I) MCF10A cells. The EVs were purified from the culture supernatants of these cells using a conventional centrifugation method. Data from each measurement are shown, revealing the overall size distribution (histograms) and mode (nm).
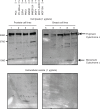
Fig. 3
Immunoblotting analysis for cytochrome c, a negative control of EVs. Proteins from whole cell lysates (upper panel) or EVs (lower panel) were separated on SDS-PAGE gels followed by Western blotting using antibodies against cytochrome c. A 1 µg sample of cell lysate and 1 µg of EV proteins were used. Lane 1: PC3 cells; lane 2: PC-3M-luc cells; lane 3: 22Rv1 cells; lane 4: PNT2 cells; lane 5: MDA-MB-231-luc-D3H1 cells (MM231); lane 6: MDA-MB-231-luc-D3H2LN cells (MM231LN); lane 7: MCF7 cells; lane 8: MCF7-ADR cells and lane 9: MCF10A cells.
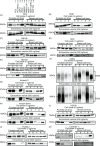
Fig. 4
Immunoblotting analysis for conventional markers of EVs derived from prostate cell lines and breast cell lines. Proteins from whole cell lysates (upper panel) or EVs (lower panel) were separated on SDS-PAGE gels, followed by Western blotting using antibodies against 11 different EV markers. A 1 µg sample of cell lysate was used for the detection of Annexin 2 and actin. The other markers were detected using 5 µg of whole cell lysate. A 250 ng sample of EV proteins was used for the detection of Annexin 2. The other markers were detected using 500 ng of EV proteins. CD63, CD9, CD81 and Integrin beta 1 were detected under non-reducing conditions. Lane 1: PC3 cells; lane 2: PC-3M-luc cells; lane 3: 22Rv1 cells; lane 4: PNT2 cells; lane 5: MDA-MB-231-luc-D3H1 cells (MM231); lane 6: MDA-MB-231-luc-D3H2LN cells (MM231LN); lane 7: MCF7 cells; lane 8: MCF7-ADR cells and lane 9: MCF10A cells.
Similar articles
- Nanoscale flow cytometry to distinguish subpopulations of prostate extracellular vesicles in patient plasma.
Padda RS, Deng FK, Brett SI, Biggs CN, Durfee PN, Brinker CJ, Williams KC, Leong HS. Padda RS, et al. Prostate. 2019 May;79(6):592-603. doi: 10.1002/pros.23764. Epub 2019 Jan 24. Prostate. 2019. PMID: 30680751 - Differential proteomics argues against a general role for CD9, CD81 or CD63 in the sorting of proteins into extracellular vesicles.
Fan Y, Pionneau C, Cocozza F, Boëlle PY, Chardonnet S, Charrin S, Théry C, Zimmermann P, Rubinstein E. Fan Y, et al. J Extracell Vesicles. 2023 Aug;12(8):e12352. doi: 10.1002/jev2.12352. J Extracell Vesicles. 2023. PMID: 37525398 Free PMC article. - Diverse Populations of Extracellular Vesicles with Opposite Functions during Herpes Simplex Virus 1 Infection.
Dogrammatzis C, Saleh S, Deighan C, Kalamvoki M. Dogrammatzis C, et al. J Virol. 2021 Feb 24;95(6):e02357-20. doi: 10.1128/JVI.02357-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361424 Free PMC article. - Ultrastructural identification of CD9 positive extracellular vesicles released from human embryos and transported through the zona pellucida.
Vyas P, Balakier H, Librach CL. Vyas P, et al. Syst Biol Reprod Med. 2019 Aug;65(4):273-280. doi: 10.1080/19396368.2019.1619858. Epub 2019 May 28. Syst Biol Reprod Med. 2019. PMID: 31136209 - A novel multiplexed immunoassay for surface-exposed proteins in plasma extracellular vesicles.
Tordoff E, Allen J, Elgart K, Elsherbini A, Kalia V, Wu H, Eren E, Kapogiannis D, Gololobova O, Witwer K, Volpert O, Eitan E. Tordoff E, et al. J Extracell Vesicles. 2024 Nov;13(11):e70007. doi: 10.1002/jev2.70007. J Extracell Vesicles. 2024. PMID: 39498678 Free PMC article.
Cited by
- Extracellular vesicles in prostate cancer: a narrative review.
Hatano K, Fujita K. Hatano K, et al. Transl Androl Urol. 2021 Apr;10(4):1890-1907. doi: 10.21037/tau-20-1210. Transl Androl Urol. 2021. PMID: 33968677 Free PMC article. Review. - Use of four genes in exosomes as biomarkers for the identification of lung adenocarcinoma and lung squamous cell carcinoma.
Cao B, Wang P, Gu L, Liu J. Cao B, et al. Oncol Lett. 2021 Apr;21(4):249. doi: 10.3892/ol.2021.12510. Epub 2021 Feb 3. Oncol Lett. 2021. PMID: 33664813 Free PMC article. - Plasma extracellular vesicles detected by Single Molecule array technology as a liquid biopsy for colorectal cancer.
Wei P, Wu F, Kang B, Sun X, Heskia F, Pachot A, Liang J, Li D. Wei P, et al. J Extracell Vesicles. 2020 Aug 26;9(1):1809765. doi: 10.1080/20013078.2020.1809765. J Extracell Vesicles. 2020. PMID: 32944195 Free PMC article. - Unraveling the Connection: Extracellular Vesicles and Non-Small Cell Lung Cancer.
Wu J, Chen Y. Wu J, et al. Int J Nanomedicine. 2024 Aug 9;19:8139-8157. doi: 10.2147/IJN.S477851. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39139506 Free PMC article. Review.
References
- Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–68. - PubMed
- van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. - PubMed
- Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–53. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous