Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis - PubMed (original) (raw)
Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis
Chris Gardiner et al. J Extracell Vesicles. 2013.
Abstract
Nanoparticle tracking analysis (NTA) is a light-scattering technique that is useful for the rapid sizing and enumeration of extracellular vesicles (EVs). As a relatively new method, NTA has been criticised for a lack of standardisation. We propose the use of silica microspheres for the calibration of NTA measurements and describe in detail a protocol for the analysis of EVs by NTA which should minimise many of the sources of variability and imprecision associated with this technique.
Keywords: extracellular vesicles; light scattering; nanoparticle tracking analysis; standardisation.
Figures
Fig. 1
Onscreen images showing (A) the correct position of the “thumbprint” at the zero position; (B) overexposed particles due to inappropriately high camera settings; (C) a correctly focussed image of an appropriate concentration of particles; poorly focussed particles due to the stage being (D) too low or (E) too high; and a sample that is too concentrated for analysis.
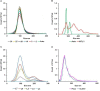
Fig. 2
(A) The effect of minimum track length (MTL) on measured size distribution of monodisperse 100 nm silica microspheres; (B) the effect of using automatic (Auto) or manual (MTL5) minimum track length on measurement of a low concentration of polydisperse EVs; (C) the effect of increasing camera level (level 3 to level 7) on the measurement of a mixture of 100 nm and 200 nm microspheres (concentration 10×108/ml and 0.5×108/ml, respectively); and (D) NTA analysis of plasma EVs labelled with CellMask using light scattering (Scatter) and fluorescence (Fluor) measurement.
Similar articles
- Enumeration of extracellular vesicles by a new improved flow cytometric method is comparable to fluorescence mode nanoparticle tracking analysis.
Pasalic L, Williams R, Siupa A, Campbell H, Henderson MJ, Chen VMY. Pasalic L, et al. Nanomedicine. 2016 May;12(4):977-986. doi: 10.1016/j.nano.2015.12.370. Epub 2016 Jan 6. Nanomedicine. 2016. PMID: 26767510 - Measurement of refractive index by nanoparticle tracking analysis reveals heterogeneity in extracellular vesicles.
Gardiner C, Shaw M, Hole P, Smith J, Tannetta D, Redman CW, Sargent IL. Gardiner C, et al. J Extracell Vesicles. 2014 Nov 24;3:25361. doi: 10.3402/jev.v3.25361. eCollection 2014. J Extracell Vesicles. 2014. PMID: 25425324 Free PMC article. - Differential fluorescence nanoparticle tracking analysis for enumeration of the extracellular vesicle content in mixed particulate solutions.
Desgeorges A, Hollerweger J, Lassacher T, Rohde E, Helmbrecht C, Gimona M. Desgeorges A, et al. Methods. 2020 May 1;177:67-73. doi: 10.1016/j.ymeth.2020.02.006. Epub 2020 Feb 17. Methods. 2020. PMID: 32081745 - Nanoparticle Tracking Analysis: An Effective Tool to Characterize Extracellular Vesicles.
Kowkabany G, Bao Y. Kowkabany G, et al. Molecules. 2024 Oct 1;29(19):4672. doi: 10.3390/molecules29194672. Molecules. 2024. PMID: 39407601 Free PMC article. Review. - Analytical challenges of extracellular vesicle detection: A comparison of different techniques.
Erdbrügger U, Lannigan J. Erdbrügger U, et al. Cytometry A. 2016 Feb;89(2):123-34. doi: 10.1002/cyto.a.22795. Epub 2015 Dec 9. Cytometry A. 2016. PMID: 26651033 Review.
Cited by
- Isolation and characterization of vesicular and non-vesicular microRNAs circulating in sera of partially hepatectomized rats.
Castoldi M, Kordes C, Sawitza I, Häussinger D. Castoldi M, et al. Sci Rep. 2016 Aug 18;6:31869. doi: 10.1038/srep31869. Sci Rep. 2016. PMID: 27535708 Free PMC article. - Exosomes and exosomal miRNAs in cardiovascular protection and repair.
Emanueli C, Shearn AI, Angelini GD, Sahoo S. Emanueli C, et al. Vascul Pharmacol. 2015 Aug;71:24-30. doi: 10.1016/j.vph.2015.02.008. Epub 2015 Apr 11. Vascul Pharmacol. 2015. PMID: 25869502 Free PMC article. Review. - Proteomic Characterization of Corneal Epithelial and Stromal Cell-Derived Extracellular Vesicles.
Yeung V, Boychev N, Kanu LN, Ng V, Ross AE, Hutcheon AEK, Ciolino JB. Yeung V, et al. Int J Mol Sci. 2024 Sep 26;25(19):10338. doi: 10.3390/ijms251910338. Int J Mol Sci. 2024. PMID: 39408670 Free PMC article. - Miniaturized Biomedical Sensors for Enumeration of Extracellular Vesicles.
Pulikkathodi AK, Sarangadharan I, Lo CY, Chen PH, Chen CC, Wang YL. Pulikkathodi AK, et al. Int J Mol Sci. 2018 Jul 29;19(8):2213. doi: 10.3390/ijms19082213. Int J Mol Sci. 2018. PMID: 30060613 Free PMC article. - Small RNA-sequence analysis of plasma-derived extracellular vesicle miRNAs in smokers and patients with chronic obstructive pulmonary disease as circulating biomarkers.
Sundar IK, Li D, Rahman I. Sundar IK, et al. J Extracell Vesicles. 2019 Nov 7;8(1):1684816. doi: 10.1080/20013078.2019.1684816. eCollection 2019. J Extracell Vesicles. 2019. PMID: 31762962 Free PMC article.
References
- Saveyn H, De BB, Thas O, Hole P, Smith J, Van der Meeren P. Accurate particle size distribution determination by nanoparticle tracking analysis based on 2-D Brownian dynamics simulation. J Colloid Interface Sci. 2010;352:593–600. - PubMed
- Van der Meeren P, Kasinos M, Saveyn H. Relevance of two-dimensional Brownian motion dynamics in applying nanoparticle tracking analysis. Methods Mol Biol. 2012;906:525–34. - PubMed
- Carr R, Hole P, Malloy A, Nelson P, Smith J. Applications of nanoparticle tracking analysis (NTA) in nanoparticle research – a mini-review. Eur J Parent Pharmaceut Sci. 2009;14:45–50.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials