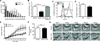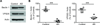Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer's disease - PubMed (original) (raw)
Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer's disease
Kurt M Lucin et al. Neuron. 2013.
Abstract
Phagocytosis controls CNS homeostasis by facilitating the removal of unwanted cellular debris. Accordingly, impairments in different receptors or proteins involved in phagocytosis result in enhanced inflammation and neurodegeneration. While various studies have identified extrinsic factors that modulate phagocytosis in health and disease, key intracellular regulators are less understood. Here we show that the autophagy protein beclin 1 is required for efficient phagocytosis in vitro and in mouse brains. Furthermore, we show that beclin 1-mediated impairments in phagocytosis are associated with dysfunctional recruitment of retromer to phagosomal membranes, reduced retromer levels, and impaired recycling of phagocytic receptors CD36 and Trem2. Interestingly, microglia isolated from human Alzheimer's disease (AD) brains show significantly reduced beclin 1 and retromer protein levels. These findings position beclin 1 as a link between autophagy, retromer trafficking, and receptor-mediated phagocytosis and provide insight into mechanisms by which phagocytosis is regulated and how it may become impaired in AD.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
Figure 1
Reduced beclin 1 impairs microglial phagocytosis. (A) BV2 microglia cells were infected with lentivirus encoding for luciferase shRNA (control) or beclin 1 shRNA (KD). Cell lysates were analyzed by western blot. Infected cells were provided fluorescent latex beads and phagocytosis was analyzed by flow cytometry (B–C). Data are expressed as phagocytic index, which is the percent of cells that phagocytose beads. (D) BV2 cells receiving beclin 1 shRNA lentivirus were transduced with lentivirus encoding for mouse beclin 1 (mBeclin 1) and cell lysates were analyzed by western blot. (E) Recovering beclin 1 levels was sufficient to improve phagocytosis of latex beads. Results were compared by an unpaired Student’s t test (C) or a one-way ANOVA with a Tukey’s post-test (E) and are representative of at least 3 independent experiments (n=3 per group). Values are mean ± SEM. *** p<0.001.
Figure 2
Reduced beclin 1 impairs microglial phagocytic efficiency. BV2 microglia cells were infected with lentivirus encoding for luciferase shRNA (control) or beclin 1 shRNA (KD). (A) Phagocytosis of fluorescent latex beads was assayed by flow cytometry and analysis of flow cytometry histograms determined the number of BV2 cells phagocytosing relative quantities of beads. (B) Recovering beclin 1 levels with a lentivirus encoding for mouse beclin 1 (mBeclin 1) was sufficient to rescue phagocytic efficiency as measured by flow cytometry. (C) Primary microglia were isolated from beclin 1+/− (Becn1+/−) or wildtype (WT) mice and labeled with fluorescently tagged antibodies against CD11b or isotype control antibodies. The percent of CD11b+ cells in the microglial population was determined by flow cytometry relative to the cells receiving isotype control antibody. The microglial population was determined to be ~90% pure. (D) The average number of beads phagocytosed by Becn1+/− and WT primary microglia was quantified by flow cytometry. (E) Using live-cell imaging the number of beads phagocytosed over time was monitored. (F) Cell motility was also monitored by live-cell imaging. (G) Shown are representative images from live-cell imaging at various times after the administration of beads. To better visualize the beads an outline is provided around each bead. Scale bar, 15 µm. For further analysis of bead uptake please see Figure S2. Results were compared by a two-way ANOVA with a Bonferroni post-test (A,E), a one-way ANOVA with a Tukey’s post-test (B), or an unpaired Student’s t test (D,F) and are representative of at least 3 independent experiments (A–B; n=3 per group, D; n=4–6 per group, E–F; n=10–16 per group). Values are mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001.
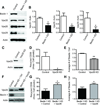
Figure 3
Reduced beclin 1 diminishes phagocytic receptor recycling and retromer localization. (A) To determine whether CD36 contributes to phagocytosis of latex beads, wildtype BV2 cells were cultured with latex beads in the presence or absence of CD36 neutralizing antibody or IgG control antibody. Phagocytosis was quantified by flow cytometry. (B–D) Using an established receptor recycling assay, CD36 receptor recycling was analyzed in BV2 cells infected with lentivirus encoding luciferase shRNA (control) or beclin 1 shRNA (KD). Scale bar, 100 µm. (E-F) CD36 receptor recycling was analyzed in Becn1+/− and WT primary microglia. The cell highlighted within the dashed box (E) is shown at a higher magnification in the insert. Scale bar, 100 ⌠m. (G) Using flow cytometry, surface expression of CD36 was determined in the absence of ligand for BV2 cells infected with beclin 1 shRNA or control lentivirus. (H) Trem2 receptor recycling was analyzed in BV2 cells infected with beclin 1 shRNA or control lentivirus. All bars are mean ± SEM; mean differences were compared by a one-way ANOVA with a Tukey’s post-test (A) or an unpaired Student’s t test (D,F–H). (A,D,G; n=3 per group, F,H; n=4–6 per group). *p<0.05, **p<0.01, n.s., not significant.
Figure 4
Beclin 1 regulates the retromer complex, which controls CD36 recycling. (A-B) BV2 cells were infected with a lentivirus encoding for luciferase shRNA (control) or beclin 1 shRNA (KD) and cell lysates were analyzed by western blot to detect levels of the retromer complex. (C) BV2 cells were infected with a lentivirus encoding for Vps35 shRNA (Vps35 KD) or luciferase shRNA (Control) and cell lysates were probed by western blot. CD36 receptor recycling (D) and phagocytic efficiency (E) were then analyzed in BV2 cells infected with Vps35 shRNA or control lentivirus. (F) To confirm our Vps35 rescue approach, HEK cells were infected with either control lentivirus, beclin 1 shRNA lentivirus (Becn KD) and transfected with a control RFP plasmid (Ctrl), or beclin 1 shRNA lentivirus and transfected with a Vps35-T2A-RFP plasmid (Vps35) and cell lysates were analyzed by western blot. CD36 receptor recycling (G) and phagocytosis (H) was impaired in BV2 cells infected with beclin 1 shRNA lentivirus and transfected with control plasmid when compared to BV2 cells infected with beclin 1 shRNA lentivirus and transfected with Vps35 plasmid. Phagocytic analysis by flow cytometry was investigated in cellular populations expressing RFP. All bars are mean ± SEM and are representative of at least two independent experiments (n=3 per group). Mean differences were compared by an unpaired Student’s t test. *p<0.05, **p<0.01, ***p<0.001.
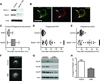
Figure 5
Reduced beclin 1 impairs Vps34-mediated recruitment of retromer. (A) Cell lysates from BV2 cells infected with lentivirus encoding for luciferase shRNA (control) or beclin 1 shRNA (KD) were analyzed by western blot. (B) BV2 cells transfected with a phosphatidylinositol 3-phosphate (PI3P) reporter (2xFYVE-RFP) were immunofluorescently labeled for Vps35 and co-localization was visualized by confocal microscopy. Arrows indicate vesicles with co-localization. Scale bar, 20µm. (C) Live-cell imaging of BV2 cells transfected with 2xFYVE-RFP or Vps35-RFP were used to monitor the kinetics by which PI3P and Vps35 localize to the phagosomal membrane of internalized 6µm latex beads. BV2 cells transfected with either 2xFYVE-RFP or Vps35-RFP were subsequently infected with beclin 1 or control shRNA lentivirus and the phagosomal localization of PI3P (D) and Vps35 (E) was similarly monitored by live-cell imaging after the internalization of 6µm latex beads. The X symbols in figures D and E represent the absence of localization in a 90min live-cell imaging session. Please see Movies S1–4 for representative examples of phagosomal localization. (F) BV2 cells transfected with Vps35-RFP were treated with 10mM of the PI3-kinase inhibitor 3-methyladenine (3MA) or PBS (control) for 3hrs and the localization of Vps35 was visualized. (G) Cell lysates from BV2 cells treated with 5mM 3MA for 48hrs were probed by western blot to detect levels of the retromer complex. (H) The average number of beads phagocytosed by BV2 cells receiving 10mM 3-MA or PBS (control) was quantified by flow cytometry. Values are mean ± SEM and are representative of at least two independent experiments (C–E; n=25–31 beads per group, H; n=3 per group).
Figure 6
Reduced microglial beclin 1 impairs Aβ phagocytosis. (A) BV2 cells were infected with beclin 1 shRNA (KD) or luciferase shRNA (control) lentivirus and cultured on 10µm thick freshly cut, unfixed brain slices from APP transgenic mice. After 24hrs the slices were processed for Aβ histology or ELISA. (B) Confocal microscopy confirmed that BV2 cells cultured on APP brain slices contained Aβ after 24hrs of culture. Transduced BV2 cells were visualized with GFP and Aβ was visualized with 3D6. Scale bar, 10µm. (C) Sections were then probed for Aβ and Aβ load was quantified in the hippocampus (D) and cortex (E). Scale bar, 200µm. Aβ was also extracted from replicate slices and total Aβ (F) and Aβ1–42 (G) were measured by ELISA. Please see Figure S4 for analysis with primary microglia. All bars are mean ± SEM. Mean differences were compared by a one-way ANOVA with a Tukey’s post-test (D,E) or an unpaired Student’s t test (F,G) and are representative of at least two independent experiments (n=6–10 per group). *p<0.05.
Figure 7
Beclin 1-deficient mice demonstrate impaired extracellular Aβ clearance and phagocytosis. (A) To determine whether beclin 1+/− mice (Becn1+/−) demonstrate deficits in extracellular Aβ phagocytosis and removal, fibrillar Aβ1–42 or pH-sensitive beads were stereotaxically injected into the frontal cortex. Brain image was obtained from Allen Brain Atlas. (B-C) Residual Aβ was quantified histologically 48hrs after injection. Scale bar, 500µm. (D-E) Fluorescence from pH-sensitive beads, which was confirmed with BV2 cells in vitro (See Insert), was quantified from confocal images 48hrs after injection. Scale bar, 200µm. (F) Primary microglia isolated from beclin 1+/− mice were analyzed by LysoSensor Yellow/Blue dextran to determine relative lysosomal pH levels. For additional measures of phagosomal and lysosomal pH please see Figure S5. Results were analyzed by an unpaired Student’s t test (n=9–11 (C,E) or n=4 (F) per group). All values are mean ± SEM. *p<0.05; n.s., non significant.
Figure 8
Microglia isolated from human AD brains show reduced levels of beclin 1 and VPS35. Human microglia isolated from the superior and middle frontal gyri of postmortem AD or non-demented control patients were lysed, the detergent soluble protein fraction was probed by western blot (A), and protein levels were quantified relative to actin (B). Results were analyzed by an unpaired Student’s t test (n=5 per group). All values are mean ± SEM ***p<0.001.
Similar articles
- Beclin 1 regulates astrocyte phagocytosis and phagosomal recruitment of retromer.
Lemus Silva EG, Delgadillo Y, White RE, Lucin KM. Lemus Silva EG, et al. Tissue Cell. 2023 Jun;82:102100. doi: 10.1016/j.tice.2023.102100. Epub 2023 Apr 28. Tissue Cell. 2023. PMID: 37182392 - Vps35-dependent recycling of Trem2 regulates microglial function.
Yin J, Liu X, He Q, Zhou L, Yuan Z, Zhao S. Yin J, et al. Traffic. 2016 Dec;17(12):1286-1296. doi: 10.1111/tra.12451. Epub 2016 Nov 1. Traffic. 2016. PMID: 27717139 - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - TREM2 alters the phagocytic, apoptotic and inflammatory response to Aβ42 in HMC3 cells.
Akhter R, Shao Y, Formica S, Khrestian M, Bekris LM. Akhter R, et al. Mol Immunol. 2021 Mar;131:171-179. doi: 10.1016/j.molimm.2020.12.035. Epub 2021 Jan 15. Mol Immunol. 2021. PMID: 33461764 Free PMC article. - Sorting through the roles of beclin 1 in microglia and neurodegeneration.
O'Brien CE, Wyss-Coray T. O'Brien CE, et al. J Neuroimmune Pharmacol. 2014 Jun;9(3):285-92. doi: 10.1007/s11481-013-9519-8. Epub 2014 Jan 3. J Neuroimmune Pharmacol. 2014. PMID: 24385262 Free PMC article. Review.
Cited by
- miR-Let7A Modulates Autophagy Induction in LPS-Activated Microglia.
Song J, Oh Y, Lee JE. Song J, et al. Exp Neurobiol. 2015 Jun;24(2):117-25. doi: 10.5607/en.2015.24.2.117. Epub 2015 Jun 17. Exp Neurobiol. 2015. PMID: 26113790 Free PMC article. - Molecular and genetic inflammation networks in major human diseases.
Zhao Y, Forst CV, Sayegh CE, Wang IM, Yang X, Zhang B. Zhao Y, et al. Mol Biosyst. 2016 Jul 19;12(8):2318-41. doi: 10.1039/c6mb00240d. Mol Biosyst. 2016. PMID: 27303926 Free PMC article. Review. - Beclin 1 regulates recycling endosome and is required for skin development in mice.
Noguchi S, Honda S, Saitoh T, Matsumura H, Nishimura E, Akira S, Shimizu S. Noguchi S, et al. Commun Biol. 2019 Jan 25;2:37. doi: 10.1038/s42003-018-0279-0. eCollection 2019. Commun Biol. 2019. PMID: 30701202 Free PMC article. - Spermidine reduces neuroinflammation and soluble amyloid beta in an Alzheimer's disease mouse model.
Freitag K, Sterczyk N, Wendlinger S, Obermayer B, Schulz J, Farztdinov V, Mülleder M, Ralser M, Houtman J, Fleck L, Braeuning C, Sansevrino R, Hoffmann C, Milovanovic D, Sigrist SJ, Conrad T, Beule D, Heppner FL, Jendrach M. Freitag K, et al. J Neuroinflammation. 2022 Jul 2;19(1):172. doi: 10.1186/s12974-022-02534-7. J Neuroinflammation. 2022. PMID: 35780157 Free PMC article. - Transcription factor EB-mediated mesenchymal stem cell therapy induces autophagy and alleviates spinocerebellar ataxia type 3 defects in neuronal cells model.
Han X, de Dieu Habimana J, Li AL, Huang R, Mukama O, Deng W, Wang L, Zhang Y, Wang W, Deng S, Peng K, Ni B, Zhang S, Huang J, Yan XX, Li Z. Han X, et al. Cell Death Dis. 2022 Jul 18;13(7):622. doi: 10.1038/s41419-022-05085-0. Cell Death Dis. 2022. PMID: 35851059 Free PMC article.
References
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG18440/AG/NIA NIH HHS/United States
- R01 AG045034/AG/NIA NIH HHS/United States
- F31 NS078865/NS/NINDS NIH HHS/United States
- T32 MH020016/MH/NIMH NIH HHS/United States
- R01 AG020603/AG/NIA NIH HHS/United States
- I01 BX001319/BX/BLRD VA/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- F31 AG040877/AG/NIA NIH HHS/United States
- U24 NS072026/NS/NINDS NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- 1 F31 AG040877-01A1/AG/NIA NIH HHS/United States
- P30 NS069375/NS/NINDS NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- R01 AG043384/AG/NIA NIH HHS/United States
- R01 AG030144/AG/NIA NIH HHS/United States
- R01 AG10435/AG/NIA NIH HHS/United States
- P30 AG19610/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases