Oncolytic viruses as therapeutic cancer vaccines - PubMed (original) (raw)
Review
Oncolytic viruses as therapeutic cancer vaccines
David L Bartlett et al. Mol Cancer. 2013.
Abstract
Oncolytic viruses (OVs) are tumor-selective, multi-mechanistic antitumor agents. They kill infected cancer and associated endothelial cells via direct oncolysis, and uninfected cells via tumor vasculature targeting and bystander effect. Multimodal immunogenic cell death (ICD) together with autophagy often induced by OVs not only presents potent danger signals to dendritic cells but also efficiently cross-present tumor-associated antigens from cancer cells to dendritic cells to T cells to induce adaptive antitumor immunity. With this favorable immune backdrop, genetic engineering of OVs and rational combinations further potentiate OVs as cancer vaccines. OVs armed with GM-CSF (such as T-VEC and Pexa-Vec) or other immunostimulatory genes, induce potent anti-tumor immunity in both animal models and human patients. Combination with other immunotherapy regimens improve overall therapeutic efficacy. Coadministration with a HDAC inhibitor inhibits innate immunity transiently to promote infection and spread of OVs, and significantly enhances anti-tumor immunity and improves the therapeutic index. Local administration or OV mediated-expression of ligands for Toll-like receptors can rescue the function of tumor-infiltrating CD8+ T cells inhibited by the immunosuppressive tumor microenvironment and thus enhances the antitumor effect. Combination with cyclophosphamide further induces ICD, depletes Treg, and thus potentiates antitumor immunity. In summary, OVs properly armed or in rational combinations are potent therapeutic cancer vaccines.
Figures
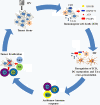
Figure 1
ICD of cancer cells induced by OVs leads to antitumor immunity. An OV, delivered either intratumorally or systemically, reaches to tumor tissue and selectively replicates in tumor or/and stromal cells. This leads to induction of death of these cells, presenting “eat me” signals on the cell surface and later release of danger signals from necrotic cells. Apoptotic bodies are engulfed by APC, and TAAs are processed and presented along with MHC complex and costimulatory molecules. The released DAMPs (and PAMPs) activate and mature DCs, and TAAs are cross-presented to naive T cells. This process can be further enhanced at different steps by other immunomodulatory agents (in a combination strategy). The resulting cytotoxic immune response against tumor and associated stromal cells, involving CD4+ and CD8+ T cells, may help in complete eradication of tumor mass. Additional immunotherapies targeting DCs, T cells, and the immunosuppressive TME can further enhance this antitumor immune response.
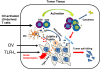
Figure 2
A model of how TILs in the TME are rescued to exert their effector functions by TLR ligands-reactivated DCs in the TME. TILs activated by oncolytic virotherapy or other cancer vaccines migrated from lymph nodes to the tumor tissues may require in situ activation by tumor–infiltrated DCs. However, the tumor-infiltrated DCs (TIDCs) are immunologically suppressed in the TME, but can be activated by TLR ligands or/and other TLR3/9 ligands (TLR ligands) through type I IFN-dependent signaling. Some OVs themselves or their products (such as dsRNA) can function as TLR ligands. The functionally reactivated TIDCs can acquire, process, and present TAAs to reactivate TILs to exert their functions. This model is modified from Xiao H et al., 2013 [153].
Similar articles
- Arming oncolytic viruses to leverage antitumor immunity.
de Gruijl TD, Janssen AB, van Beusechem VW. de Gruijl TD, et al. Expert Opin Biol Ther. 2015 Jul;15(7):959-71. doi: 10.1517/14712598.2015.1044433. Epub 2015 May 10. Expert Opin Biol Ther. 2015. PMID: 25959450 Review. - Oncolytic viruses and their application to cancer immunotherapy.
Chiocca EA, Rabkin SD. Chiocca EA, et al. Cancer Immunol Res. 2014 Apr;2(4):295-300. doi: 10.1158/2326-6066.CIR-14-0015. Cancer Immunol Res. 2014. PMID: 24764576 Free PMC article. Review. - Critical Interactions between Immunogenic Cancer Cell Death, Oncolytic Viruses, and the Immune System Define the Rational Design of Combination Immunotherapies.
van Vloten JP, Workenhe ST, Wootton SK, Mossman KL, Bridle BW. van Vloten JP, et al. J Immunol. 2018 Jan 15;200(2):450-458. doi: 10.4049/jimmunol.1701021. J Immunol. 2018. PMID: 29311387 Review. - Armed oncolytic viruses: A kick-start for anti-tumor immunity.
de Graaf JF, de Vor L, Fouchier RAM, van den Hoogen BG. de Graaf JF, et al. Cytokine Growth Factor Rev. 2018 Jun;41:28-39. doi: 10.1016/j.cytogfr.2018.03.006. Epub 2018 Mar 19. Cytokine Growth Factor Rev. 2018. PMID: 29576283 Free PMC article. Review. - Oncolytic ImmunoViroTherapy: A long history of crosstalk between viruses and immune system for cancer treatment.
Feola S, Russo S, Ylösmäki E, Cerullo V. Feola S, et al. Pharmacol Ther. 2022 Aug;236:108103. doi: 10.1016/j.pharmthera.2021.108103. Epub 2021 Dec 23. Pharmacol Ther. 2022. PMID: 34954301 Review.
Cited by
- Viruses in glioblastoma: an update on evidence and clinical trials.
Gunasegaran B, Ashley CL, Marsh-Wakefield F, Guillemin GJ, Heng B. Gunasegaran B, et al. BJC Rep. 2024 Apr 19;2(1):33. doi: 10.1038/s44276-024-00051-z. BJC Rep. 2024. PMID: 39516641 Free PMC article. Review. - Harnessing the Therapeutic Potential of Mesenchymal Stem Cells in Cancer Treatment.
Kangari P, Salahlou R, Vandghanooni S. Kangari P, et al. Adv Pharm Bull. 2024 Oct;14(3):574-590. doi: 10.34172/apb.2024.052. Epub 2024 Jun 22. Adv Pharm Bull. 2024. PMID: 39494266 Free PMC article. Review. - Oncolytic Viruses as Reliable Adjuvants in CAR-T Cell Therapy for Solid Tumors.
Stilpeanu RI, Secara BS, Cretu-Stancu M, Bucur O. Stilpeanu RI, et al. Int J Mol Sci. 2024 Oct 16;25(20):11127. doi: 10.3390/ijms252011127. Int J Mol Sci. 2024. PMID: 39456909 Free PMC article. Review. - Cancer immunotherapy and its facilitation by nanomedicine.
Sui C, Wu H, Li X, Wang Y, Wei J, Yu J, Wu X. Sui C, et al. Biomark Res. 2024 Aug 3;12(1):77. doi: 10.1186/s40364-024-00625-6. Biomark Res. 2024. PMID: 39097732 Free PMC article. Review. - Analysis of physical and biological delivery systems for DNA cancer vaccines and their translation to clinical development.
Oelkrug C. Oelkrug C. Clin Exp Vaccine Res. 2024 Apr;13(2):73-82. doi: 10.7774/cevr.2024.13.2.73. Epub 2024 Apr 30. Clin Exp Vaccine Res. 2024. PMID: 38752006 Free PMC article. Review.
References
- Cerullo V, Koski A, Vaha-Koskela M, Hemminki A. Chapter eight–Oncolytic adenoviruses for cancer immunotherapy: data from mice, hamsters, and humans. Adv Cancer Res. 2012;115:265–318. - PubMed
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA, Stojdl DF, Daneshmand M, Speth K, Kirn D. et al.Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–1693. - PubMed
- Liu TC, Hwang T, Park BH, Bell J, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–1642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01CA132714/CA/NCI NIH HHS/United States
- T32CA113263-01/CA/NCI NIH HHS/United States
- T32 CA113263/CA/NCI NIH HHS/United States
- R01CA16444/CA/NCI NIH HHS/United States
- R01CA155925/CA/NCI NIH HHS/United States
- R01 CA155925/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials