Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate - PubMed (original) (raw)
Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate
Kevin M Dickson et al. Biochem Biophys Res Commun. 2013.
Abstract
Tet (ten-eleven translocation) methylcytosine dioxygenases, which belong to the iron and 2-oxoglutarate (2OG)-dependent dioxygenase superfamily, convert 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) in DNA. We recently reported that ascorbate (vitamin C) induces Tet-mediated generation of 5hmC. To initially delineate the role of ascorbate on 5hmC generation, we analyzed whether the effect of ascorbate is dependent upon the conditions of other components involved in the hydroxylation of 5mC catalyzed by Tet. We found that removing iron from the culture medium did not affect the induction of 5hmC by ascorbate (10 μM) in mouse embryonic fibroblasts (MEFs). The effect of ascorbate did not involve an increased expression of Tet1-3 or isocitrate dehydrogenases (IDH1-2), the enzymes responsible for producing 2OG. Interestingly, MEFs cultured with different concentrations of glucose, a major precursor of 2OG, exhibited nearly identical responses to ascorbate treatment. Further, blocking the uptake of the reduced form of vitamin C, ascorbic acid, through the sodium-dependent vitamin C transporters (SVCTs) inhibited the effect of ascorbate on 5hmC. However, inhibition of the facilitative glucose transporters (GLUTs), which mediate the incorporation of the oxidized form of vitamin C, dehydroascorbic acid (DHA), did not modify the ability of ascorbate to induce 5hmC generation. These results indicate that the effect of ascorbate on 5hmC is not dependent upon iron uptake, the expression of Tet and IDH, or the production of 2OG, suggesting that ascorbate may directly participate in the generation of 5hmC, most likely as a cofactor of Tet.
Keywords: 2-Oxoglutarate; 5-Hydroxymethylcytosine; Ascorbate; Glucose; Iron; Isocitrate dehydrogenase; Sodium-dependent vitamin C transporter; Tet methylcytosine dioxygenase.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Fig. 1
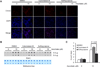
The generation of 5hmC induced by ascorbate is independent on the iron uptake by MEFs. (A) Immunostaining shows that the 5hmC signal induced by ascorbate (10 µM) treatments for 24 hr is at a similar level in MEFs cultured with different concentrations (0, 0.25, 2.5µM) of iron. (B) A representative dot-blot shows that ascorbate (10 µM) increases the content of 5hmC in MEFs cultured with different concentrations (0, 0.25, 2.5µM) of iron. (C) Semiquantitative analysis of the dot-blot indicates that ascorbate (10 µM) increases 5hmC at a similar level (~4 fold of the basal level) in MEFs cultured with different concentrations (0, 0.25, 2.5µM) of iron. (P > 0.05 assessed by t test. Date are represented as mean ± SEM).
Fig. 2
Glucose in the medium does not change the generation of 5hmC induced by ascorbate treatment. (A) Immunostaining shows that glucose (0, 5.56, 25 mM) does not change 5hmC signal induced by ascorbate (10 µM) treatment for 24 hr. (B) A representative dot-blot shows that ascorbate (10 µM) increases the content of 5hmC in MEFs cultured with different concentrations (0, 5.56, 25 mM) of glucose. (C) Semiquantitative analysis of the dot-blot indicates that ascorbate (10 µM) increases 5hmC at a similar level (~4 fold of the basal level) in MEFs cultured with different concentrations (0, 5.56, 25 mM) of glucose. (P > 0.05 assessed by t test. Date are represented as mean ± SEM).
Fig. 3
Ascorbate treatment does not change the expression of Tet and IDH genes. (A) Quantitative PCR shows that ascorbate (0 – 1,000 µM) does not significantly change the expression of Tet genes in MEFs. (P > 0.05 assessed by t test. Date are represented as mean ± SEM). (B) Quantitative PCR shows that ascorbate (0 – 1,000 µM) does not significantly change the expression of IDH genes in MEFs. (P > 0.05 assessed by t test. Date are represented as mean ± SEM).
Fig. 4
The effect of ascorbate on 5hmC is partially reduced by sulfinpyrazone. (A) Immunostaining shows that both sulfinpyrazone (2 mM) and cytochalasin B (20 µM) do not change 5hmC signal in ascorbate-free MEFs. Only sulfinpyrazone blocks the induction of 5hmC by ascorbate (10 µM) treatment for 24 hr. (B) A representative dot-blot shows that sulfinpyrazone (2 mM), but not cytochalasin B (20 µM), inhibits the effects of ascorbate (10 µM) on 5hmC content. (C) Semiquantitative analysis of the dot-blot indicates that sulfinpyrazone (2 mM), but not cytochalasin B (20 µM), inhibits the induction of 5hmC by ascorbate (* P < 0.05 assessed by t test. Date are represented as mean ± SEM).
Similar articles
- Connections between TET proteins and aberrant DNA modification in cancer.
Huang Y, Rao A. Huang Y, et al. Trends Genet. 2014 Oct;30(10):464-74. doi: 10.1016/j.tig.2014.07.005. Epub 2014 Aug 14. Trends Genet. 2014. PMID: 25132561 Free PMC article. Review. - Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine.
Minor EA, Court BL, Young JI, Wang G. Minor EA, et al. J Biol Chem. 2013 May 10;288(19):13669-74. doi: 10.1074/jbc.C113.464800. Epub 2013 Apr 2. J Biol Chem. 2013. PMID: 23548903 Free PMC article. - Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells.
Blaschke K, Ebata KT, Karimi MM, Zepeda-Martínez JA, Goyal P, Mahapatra S, Tam A, Laird DJ, Hirst M, Rao A, Lorincz MC, Ramalho-Santos M. Blaschke K, et al. Nature. 2013 Aug 8;500(7461):222-6. doi: 10.1038/nature12362. Epub 2013 Jun 30. Nature. 2013. PMID: 23812591 Free PMC article. - Nuclear exclusion of TET1 is associated with loss of 5-hydroxymethylcytosine in IDH1 wild-type gliomas.
Müller T, Gessi M, Waha A, Isselstein LJ, Luxen D, Freihoff D, Freihoff J, Becker A, Simon M, Hammes J, Denkhaus D, zur Mühlen A, Pietsch T, Waha A. Müller T, et al. Am J Pathol. 2012 Aug;181(2):675-83. doi: 10.1016/j.ajpath.2012.04.017. Epub 2012 Jun 9. Am J Pathol. 2012. PMID: 22688054 - Tet family of 5-methylcytosine dioxygenases in mammalian development.
Zhao H, Chen T. Zhao H, et al. J Hum Genet. 2013 Jul;58(7):421-7. doi: 10.1038/jhg.2013.63. Epub 2013 May 30. J Hum Genet. 2013. PMID: 23719188 Free PMC article. Review.
Cited by
- Reduced mRNA and Protein Expression Levels of Tet Methylcytosine Dioxygenase 3 in Endothelial Progenitor Cells of Patients of Type 2 Diabetes With Peripheral Artery Disease.
Zhao S, Jia T, Tang Y, Zhang X, Mao H, Tian X, Li R, Ma L, Chen G. Zhao S, et al. Front Immunol. 2018 Dec 6;9:2859. doi: 10.3389/fimmu.2018.02859. eCollection 2018. Front Immunol. 2018. PMID: 30574144 Free PMC article. - Decreased Nuclear Ascorbate Accumulation Accompanied with Altered Genomic Methylation Pattern in Fibroblasts from Arterial Tortuosity Syndrome Patients.
Németh CE, Nemoda Z, Lőw P, Szabó P, Horváth EZ, Willaert A, Boel A, Callewaert BL, Coucke PJ, Colombi M, Bánhegyi G, Margittai É. Németh CE, et al. Oxid Med Cell Longev. 2019 Jan 13;2019:8156592. doi: 10.1155/2019/8156592. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30800210 Free PMC article. - Vitamin C regulates Schwann cell myelination by promoting DNA demethylation of pro-myelinating genes.
Huff TC, Sant DW, Camarena V, Van Booven D, Andrade NS, Mustafi S, Monje PV, Wang G. Huff TC, et al. J Neurochem. 2021 Jun;157(6):1759-1773. doi: 10.1111/jnc.15015. Epub 2020 Apr 14. J Neurochem. 2021. PMID: 32219848 Free PMC article. - Connections between TET proteins and aberrant DNA modification in cancer.
Huang Y, Rao A. Huang Y, et al. Trends Genet. 2014 Oct;30(10):464-74. doi: 10.1016/j.tig.2014.07.005. Epub 2014 Aug 14. Trends Genet. 2014. PMID: 25132561 Free PMC article. Review. - DNA Demethylation of the Foxp3 Enhancer Is Maintained through Modulation of Ten-Eleven-Translocation and DNA Methyltransferases.
Nair VS, Song MH, Ko M, Oh KI. Nair VS, et al. Mol Cells. 2016 Dec;39(12):888-897. doi: 10.14348/molcells.2016.0276. Epub 2016 Dec 13. Mol Cells. 2016. PMID: 27989104 Free PMC article.
References
- McDonough MA, Loenarz C, Chowdhury R, Clifton IJ, Schofield CJ. Structural studies on human 2OGdependent oxygenases. Curr Opin Struct Biol. 2010;20:659–672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous