Diabetes insipidus contributes to traumatic brain injury pathology via CD36 neuroinflammation - PubMed (original) (raw)
Diabetes insipidus contributes to traumatic brain injury pathology via CD36 neuroinflammation
Theo Diamandis et al. Med Hypotheses. 2013 Nov.
Abstract
Each year, over one million people in the United States are affected by traumatic brain injury (TBI). Symptoms of both acute and chronic neuroinflammation follow TBI, coinciding with a robust immune response and activation of the brain's endogenous repair mechanisms. TBI can lead to endocrine failure as a result of damage to the thalamic region of the brain, evidenced by excessive thirst and polyuria often accompanying TBI. These symptoms indicate the presence of diabetes insipidus (DI), a disruption of water homeostasis due to antidiuretic hormone deficiency. This deficiency accompanies a mechanical or neuroinflammatory damage to the thalamic region during TBI, evidenced by increased expression of inflammatory microglial marker MHCII in this brain region. Excessive thirst and urinations, which are typical DI symptoms, in our chronic TBI rats also suggest a close connection between TBI and DI. We seek to bridge this gap between TBI and DI through investigation of the Cluster of Differentiation 36 (CD36) receptor. This receptor is associated with Low-Density Lipoprotein (LDL) deregulation, pro-inflammatory events, and innate immunity regulation. We posit that CD36 exacerbates TBI through immune activation and subsequent neuroinflammation. Indeed, scientific evidence already supports pathological interaction of CD36 in other neurological disorders including stroke and Alzheimer's disease. We propose that DI contributes to TBI pathology via CD36 neuroinflammation. Use of CD36 as a biomarker may provide insights into treatment and disease pathology of TBI and DI. This unexplored avenue of research holds potential for a better understanding and treatment of TBI and DI.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
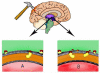
Figure 1. CD36 neuroinflammation in the thalamic/hypothalamic region - a pathological link between DI and TBI
(A). This picture depicts a brain that has suffered TBI, demonstrating a modest level of inflammation. (B). A person who suffers from diabetes insipidus has more ligands available that activate CD36, consequently causing more inflammation.
Figure 2. CD36 expression in TBI rat brain
A representative microphotograph (20x) showing activated microglial cells in the thalamus/hypothalamus of an animal subjected at 30 days post-injury after controlled cortical impact model of TBI. MHCII+ stained cells (black arrows in A), with adjacent coronal section demonstrating CD36+ (white arrows in B).
Similar articles
- Insulin-associated neuroinflammatory pathways as therapeutic targets for traumatic brain injury.
Cerecedo-López CD, Kim-Lee JH, Hernandez D, Acosta SA, Borlongan CV. Cerecedo-López CD, et al. Med Hypotheses. 2014 Feb;82(2):171-4. doi: 10.1016/j.mehy.2013.11.028. Epub 2013 Dec 1. Med Hypotheses. 2014. PMID: 24332562 Free PMC article. - Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats.
Mayeux JP, Teng SX, Katz PS, Gilpin NW, Molina PE. Mayeux JP, et al. Behav Brain Res. 2015 Feb 15;279:22-30. doi: 10.1016/j.bbr.2014.10.053. Epub 2014 Nov 10. Behav Brain Res. 2015. PMID: 25446758 Free PMC article. - Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury.
Chen X, Wu S, Chen C, Xie B, Fang Z, Hu W, Chen J, Fu H, He H. Chen X, et al. J Neuroinflammation. 2017 Jul 24;14(1):143. doi: 10.1186/s12974-017-0917-3. J Neuroinflammation. 2017. PMID: 28738820 Free PMC article. - Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention.
Kumar A, Loane DJ. Kumar A, et al. Brain Behav Immun. 2012 Nov;26(8):1191-201. doi: 10.1016/j.bbi.2012.06.008. Epub 2012 Jun 21. Brain Behav Immun. 2012. PMID: 22728326 Review. - Posterior pituitary dysfunction following traumatic brain injury: review.
Tudor RM, Thompson CJ. Tudor RM, et al. Pituitary. 2019 Jun;22(3):296-304. doi: 10.1007/s11102-018-0917-z. Pituitary. 2019. PMID: 30334138 Review.
Cited by
- Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities.
Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV. Lozano D, et al. Neuropsychiatr Dis Treat. 2015 Jan 8;11:97-106. doi: 10.2147/NDT.S65815. eCollection 2015. Neuropsychiatr Dis Treat. 2015. PMID: 25657582 Free PMC article. Review. - Alzheimer's disease and chronic traumatic encephalopathy: Distinct but possibly overlapping disease entities.
Turner RC, Lucke-Wold BP, Robson MJ, Lee JM, Bailes JE. Turner RC, et al. Brain Inj. 2016;30(11):1279-1292. doi: 10.1080/02699052.2016.1193631. Epub 2016 Aug 11. Brain Inj. 2016. PMID: 27715315 Free PMC article. Review. - Neuroinflammation in traumatic brain injury: A chronic response to an acute injury.
Schimmel SJ, Acosta S, Lozano D. Schimmel SJ, et al. Brain Circ. 2017 Jul-Sep;3(3):135-142. doi: 10.4103/bc.bc_18_17. Epub 2017 Oct 12. Brain Circ. 2017. PMID: 30276315 Free PMC article. Review. - Neuroinflammatory responses to traumatic brain injury.
Paiva WS, Correia AD, Marie SK. Paiva WS, et al. Neuropsychiatr Dis Treat. 2015 Mar 19;11:773-6. doi: 10.2147/NDT.S82109. eCollection 2015. Neuropsychiatr Dis Treat. 2015. PMID: 25834452 Free PMC article. No abstract available.
References
- Faul M, Xu L, Wald MM, Coronado VG. [Accessed July 14, 2013];Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. 2010 http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf.
- Hannon MJ, Finucane FM, Sherlock M, Agha A, Thompson CJ. Disorders of water homeostasis in Neurosurgical Patients. J Clin Endocrinol Metab. 2012;97(5):1423–33. - PubMed
- Agha A, Sherlock M, Phillips J, Tormey W, Thompson CJ. The natural history of post-traumatic neurohypophysial dysfunction. Eur J Endocrinol. 2005;52(3):371–7. - PubMed
- Tsagarkis S, Tzanela M, Dimopoulou I. Diabetes insipidus, secondary hypoadenalism and hypothyroidism after traumatic brain injury: clinical implications. Pituitary. 2005;8(3-4):251–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous