Authorship bias in violence risk assessment? A systematic review and meta-analysis - PubMed (original) (raw)
Review
Authorship bias in violence risk assessment? A systematic review and meta-analysis
Jay P Singh et al. PLoS One. 2013.
Abstract
Various financial and non-financial conflicts of interests have been shown to influence the reporting of research findings, particularly in clinical medicine. In this study, we examine whether this extends to prognostic instruments designed to assess violence risk. Such instruments have increasingly become a routine part of clinical practice in mental health and criminal justice settings. The present meta-analysis investigated whether an authorship effect exists in the violence risk assessment literature by comparing predictive accuracy outcomes in studies where the individuals who designed these instruments were study authors with independent investigations. A systematic search from 1966 to 2011 was conducted using PsycINFO, EMBASE, MEDLINE, and US National Criminal Justice Reference Service Abstracts to identify predictive validity studies for the nine most commonly used risk assessment tools. Tabular data from 83 studies comprising 104 samples was collected, information on two-thirds of which was received directly from study authors for the review. Random effects subgroup analysis and metaregression were used to explore evidence of an authorship effect. We found a substantial and statistically significant authorship effect. Overall, studies authored by tool designers reported predictive validity findings around two times higher those of investigations reported by independent authors (DOR=6.22 [95% CI=4.68-8.26] in designers' studies vs. DOR=3.08 [95% CI=2.45-3.88] in independent studies). As there was evidence of an authorship effect, we also examined disclosure rates. None of the 25 studies where tool designers or translators were also study authors published a conflict of interest statement to that effect, despite a number of journals requiring that potential conflicts be disclosed. The field of risk assessment would benefit from routine disclosure and registration of research studies. The extent to which similar conflict of interests exists in those developing risk assessment guidelines and providing expert testimony needs clarification.
Conflict of interest statement
Competing Interests: All authors are occasionally hired as experts for giving talks or workshops about risk assessment. Typically, this is done as part of the authors' regular university duties (e.g. teaching students) but depending on the nature of the task and constituents, such activities are sometimes commissioned with remuneration extraneous to their academic salaries. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures
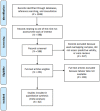
Figure 1. Results of a Systematic Search Conducted to Identify Replication Studies of Commonly Used Risk Assessment Tools.
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Correlation analysis of financial conflicts of interest and favourability of results or conclusions in addiction medicine systematic reviews and meta-analysis.
Vassar M, Shepard S, Demla S, Tritz D. Vassar M, et al. BMJ Open. 2022 Aug 29;12(8):e054325. doi: 10.1136/bmjopen-2021-054325. BMJ Open. 2022. PMID: 36038178 Free PMC article. - The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834 - A comparative study of violence risk assessment tools: a systematic review and metaregression analysis of 68 studies involving 25,980 participants.
Singh JP, Grann M, Fazel S. Singh JP, et al. Clin Psychol Rev. 2011 Apr;31(3):499-513. doi: 10.1016/j.cpr.2010.11.009. Epub 2010 Dec 13. Clin Psychol Rev. 2011. PMID: 21255891 Review. - Use of risk assessment instruments to predict violence and antisocial behaviour in 73 samples involving 24 827 people: systematic review and meta-analysis.
Fazel S, Singh JP, Doll H, Grann M. Fazel S, et al. BMJ. 2012 Jul 24;345:e4692. doi: 10.1136/bmj.e4692. BMJ. 2012. PMID: 22833604 Free PMC article. Review.
Cited by
- Are there researcher allegiance effects in diagnostic validation studies of the PHQ-9? A systematic review and meta-analysis.
Manea L, Boehnke JR, Gilbody S, Moriarty AS, McMillan D. Manea L, et al. BMJ Open. 2017 Sep 29;7(9):e015247. doi: 10.1136/bmjopen-2016-015247. BMJ Open. 2017. PMID: 28965089 Free PMC article. Review. - Prediction of violent crime on discharge from secure psychiatric hospitals: A clinical prediction rule (FoVOx).
Wolf A, Fanshawe TR, Sariaslan A, Cornish R, Larsson H, Fazel S. Wolf A, et al. Eur Psychiatry. 2018 Jan;47:88-93. doi: 10.1016/j.eurpsy.2017.07.011. Epub 2017 Aug 4. Eur Psychiatry. 2018. PMID: 29161680 Free PMC article. - Risk assessment tools in criminal justice and forensic psychiatry: The need for better data.
Douglas T, Pugh J, Singh I, Savulescu J, Fazel S. Douglas T, et al. Eur Psychiatry. 2017 May;42:134-137. doi: 10.1016/j.eurpsy.2016.12.009. Epub 2016 Dec 28. Eur Psychiatry. 2017. PMID: 28371726 Free PMC article. - The HCR-20 and violence risk assessment - will a peak of inflated expectations turn to a trough of disillusionment?
Silva E. Silva E. BJPsych Bull. 2020 Dec;44(6):269-271. doi: 10.1192/bjb.2020.14. BJPsych Bull. 2020. PMID: 33213557 Free PMC article. - Mortality, Rehospitalisation and Violent Crime in Forensic Psychiatric Patients Discharged from Hospital: Rates and Risk Factors.
Fazel S, Wolf A, Fimińska Z, Larsson H. Fazel S, et al. PLoS One. 2016 May 19;11(5):e0155906. doi: 10.1371/journal.pone.0155906. eCollection 2016. PLoS One. 2016. PMID: 27196309 Free PMC article.
References
- Perlis RH, Perlis CS, Wu Y, Hwang C, Joseph M, et al. (2005) Industry sponsorship and financial conflict of interest in the reporting of clinical trials in psychiatry. Am J Psychiatry 162: 1957–1960. - PubMed
- Bekelman JE, Li Y, Gross CP (2003) Scope and impact of financial conflicts of interest in biomedical research: A systematic review. JAMA 289: 454–465. - PubMed
- Singh JP (2012) The history, development, and testing of forensic risk assessment tools. In: Grigorenko E, editor. Handbook of juvenile forensic psychology and psychiatry. New York: Springer.
- Singh JP, Fazel S (2010) Forensic risk assessment: A metareview. Crim Justice Behav 37: 965–988.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials