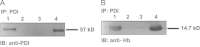Protein disulfide isomerase may facilitate the efflux of nitrite derived S-nitrosothiols from red blood cells - PubMed (original) (raw)
Protein disulfide isomerase may facilitate the efflux of nitrite derived S-nitrosothiols from red blood cells
Vasantha Madhuri Kallakunta et al. Redox Biol. 2013.
Abstract
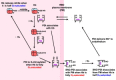
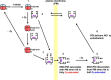
Protein disulfide isomerase (PDI) is an abundant protein primarily found in the endoplasmic reticulum and also secreted into the blood by a variety of vascular cells. The evidence obtained here, suggests that PDI could directly participate in the efflux of NO(+) from red blood cells (RBC). PDI was detected both in RBC membranes and in the cytosol. PDI was S-nitrosylated when RBCs were exposed to nitrite under ∼50% oxygen saturation but not under ∼100% oxygen saturation. Furthermore, it was observed that hemoglobin (Hb) could promote PDI S-nitrosylation in the presence of ∼600 nM nitrite. In addition, three lines of evidence were obtained for PDI-Hb interactions: (1) Hb co-immunoprecipitated with PDI; (2) Hb quenched the intrinsic PDI fluorescence in a saturable manner; and (3) Hb-Fe(II)-NO absorption spectrum decreased in a [PDI]-dependent manner. Finally, PDI was detected on the surface RBC under ∼100% oxygen saturation and released as soluble under ∼50% oxygen saturation. The soluble PDI detected under ∼50% oxygen saturation was S-nitrosylated. Based on these data it is proposed that PDI is taken up by RBC and forms a complex with Hb. Hb-Fe(II)-NO that is formed from nitrite reduction under ∼50% O2, then transfers NO(+) to either Hb-Cys β93 or directly to PDI resulting in S-nitroso-PDI which transverses the RBC membrane and attaches to the RBC surface. When RBCs enter tissues the S-nitroso-PDI is released from the RBC-surface into the blood where its NO(+) is transferred into the endothelium thereby inducing vasodilation, suggesting local oxygen-dependent dynamic interplays between nitrite, NO and S-nitrosylation.
Keywords: BCA, bicinchoninic acid; EDTA, ethylenediaminetetraacetic acid; Hb, hemoglobin; Hypoxic vasodilation; NOx, nitric oxide related species; NP-40, nonyl phenoxypolyethoxylethanol; Nitrite reductase; PDI, protein disulfide isomerase; PMSF, penylmethylsulfenylfluoride; Protein disulfide isomerase; RBC, red blood cells; Red blood cells; S-nitroso-protein disulfide isomerase; S-nitrosohemoglobin; SDS-PAGE, sodium dodecyl sulfate, poly acrylamide gel electrophoresis; SNO, S-nitrosothiol; SNO-Hb, S-nitrosohemoglobin.
Figures
Graphical abstract
Fig. 1
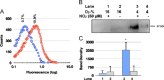
Western immunoblots of: RBC membrane fraction-Lane 1; RBC homogenate-Lane 2; and standard human PDI-Lane 3, all probed with anti-PDI primary antibodies.
Fig. 2
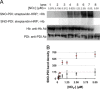
Nitrite promotes RBC-PDI _S_-nitrosylation under normoxia but not under hypoxia: (A) Freshly isolated RBCs were equilibrated with either 4% O2 (Lane 1) or 16% O2 (Lane 3) in septa sealed vials nitrite (600 nM) was introduced and incubated for 10 min. The RBCs were lysed and the S-nitrosylation status of RBC–PDI was determined by the biotin switch assay visualized with streptavidin-HRP. The band corresponding to PDI was identified from the electrophoretic mobility of standard human PDI subjected to SDS-PAGE under identical conditions (Lane 2). (B) Digitized blot densities (ImageJ) of the bands obtained from 3 different experiments with conditions identical to A in each lane. Error bars represent standard deviation (_n_=3).
Fig. 3
Hb promotes NO2−-dependent nitrosylation of PDI under normoxic conditions (16%–O2): (A) These experiments were performed using constant PDI (1 μM), Hb (0.6 mM) and varying amounts of nitrite (78 nM–5 μM) in PBS-Lanes 1–7. The headspace of the vial contained 16% O2. The mixtures were incubated at 37 °C for 10 min. Aliquots were then removed and added to cold acetone and prepared for either the SNO–PDI determination by the biotin switch assay-visualized by streptavidin-HRP or detecting HB or PDI by Western immunoblots utilizing anti-Hb or anti-PDI, respectively as the primary antibodies. Lane 8-only contained PDI (1 μM) plus nitrite (5 μM) and no Hb. (B) Digitized blot densities (ImageJ) of SNO-PDI as a function of [NO2−] in the presence of Hb (red circles) and absence of Hb (black circles). Error bars represent standard deviation (_n_=3).
Fig. 4
Hb co-immunoprecipitates with PDI: RBC were immunoprecipitated with anti-PDI:ProteinA/G-Agarose beads and immunoprecipited proteins and various controls were immunoblotted with either anti-PDI (A) or anti-Hb (B) primary antibodies: (A) Lane 1: PDI control; Lane 2: anti-PDI:protein A/G agarose; Lane 3: protein A/G agarose; Lane 4: immunoprecipitation product; (B) Lane 1: Hb control; Lane 2: anti-PDI:protein A/G agarose; Lane 3: protein A/G agarose; Lane 4: immunoprecipitation product.
Fig. 5
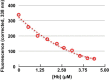
PDI intrinsic fluorescence is quenched by Hb: The intrinsic Trp fluorescence (_λ_ex 278 nm, _λ_em 339 nm) of PDI (0.75 μM) was monitored as a function of [Hb(III)]. The fluorescence was corrected for inner-filter effects by measuring the absorbance of the solution after each addition of Hb and using the equation. Fcorrected=F/1−10A(280nm).
Fig. 6
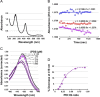
PDI denitrosates Hb-NO: (A) Hb-(II)–NO was formed by incubating dithionite-reduced Hb(II) (10.8 μM) with nitrite resulting in the characteristic Hb-(II)–NO UV/vis spectrum; (B) The Hb(II)–NO was monitored spectrophotometrically, with respect to time, at 418 nm under Ar (blue circles); or in the presence of air (500 μL ) (red circles); or in the presence of PDI (3.4 μM) plus of air (500 μL) (purple circles). The Hb-NO extinction coefficient used to convert Δ_A_(418 nm) to [Hb-NO] was 130,000 M−1 cm−1**;** (C) Hb-(II)–NO (10.8 μM) spectrum in the presence of air (500 μL ) was recorded 10 min after incubation with and varying concentration of PDI to give Hb-NO:PDI ratio between 0 and 10; **(**D) The re-plot of Δ_A_(418 nm) from C, corrected for the absorbance decrease by air alone, as a function of PDI:Hb-(II)-NO ratio.
Fig. 7
PDI associates with the RBC surface in an O2 and nitrite-dependent manner: (A) Representative flow cytometer RBC population distributions, probed for extracellular PDI. The RBCs (∼106 cells/mL) suspended in PBS were equilibrated in 16% O2 with either no nitrite (blue circles) or 50 μM nitrite (red circles) for 30 min then probed with mouse monoclonal anti-PDI antibody and sheep anti-mouse IgG-FITC and analyzed by flow cytometry; The results obtained for 4% O2 (not graphically displayed) were −nitrite 0.40%±0.10%, +nitrite 0.35%±0.12%; for 16% O2 were −nitrite 0.70%±0.085%, +nitrite 8.6%±3.8% (S.D., _n_=4); (B) Representative immunoblots of soluble PDI detected in the RBC suspension buffer by immunoprecipitation of the RBCs exposed to either 16% or 4% O2±nitrite. The immunoprecipitation product was subjected to SDS-PAGE and immunoblotted with anti-PDI primary antibodies. (D) Digitized blot densities (ImageJ) of the immunoblots (B) error bars represent S.D. (_n_=4).
Scheme 1
.
Similar articles
- Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via _β_Cys93.
Hausladen A, Qian Z, Zhang R, Premont RT, Stamler JS. Hausladen A, et al. J Pharmacol Exp Ther. 2022 Jul;382(1):1-10. doi: 10.1124/jpet.122.001194. Epub 2022 May 5. J Pharmacol Exp Ther. 2022. PMID: 35512801 Free PMC article. - An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate.
Angelo M, Singel DJ, Stamler JS. Angelo M, et al. Proc Natl Acad Sci U S A. 2006 May 30;103(22):8366-71. doi: 10.1073/pnas.0600942103. Epub 2006 May 22. Proc Natl Acad Sci U S A. 2006. PMID: 16717191 Free PMC article. - Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Circ Res. 2020 Jan 3;126(1):129-158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019 Oct 8. Circ Res. 2020. PMID: 31590598 Free PMC article. Review. - Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation.
Allen BW, Stamler JS, Piantadosi CA. Allen BW, et al. Trends Mol Med. 2009 Oct;15(10):452-60. doi: 10.1016/j.molmed.2009.08.002. Epub 2009 Sep 24. Trends Mol Med. 2009. PMID: 19781996 Free PMC article. Review. - SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation.
Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. Isbell TS, et al. Nat Med. 2008 Jul;14(7):773-7. doi: 10.1038/nm1771. Epub 2008 May 30. Nat Med. 2008. PMID: 18516054 Free PMC article.
Cited by
- Protein Transnitrosylation Signaling Networks Contribute to Inflammaging and Neurodegenerative Disorders.
Nakamura T, Oh CK, Zhang X, Tannenbaum SR, Lipton SA. Nakamura T, et al. Antioxid Redox Signal. 2021 Sep 1;35(7):531-550. doi: 10.1089/ars.2021.0081. Epub 2021 Jun 21. Antioxid Redox Signal. 2021. PMID: 33957758 Free PMC article. Review. - Mapping Soluble Guanylyl Cyclase and Protein Disulfide Isomerase Regions of Interaction.
Heckler EJ, Kholodovych V, Jain M, Liu T, Li H, Beuve A. Heckler EJ, et al. PLoS One. 2015 Nov 30;10(11):e0143523. doi: 10.1371/journal.pone.0143523. eCollection 2015. PLoS One. 2015. PMID: 26618351 Free PMC article. - Native structure of the RhopH complex, a key determinant of malaria parasite nutrient acquisition.
Ho CM, Jih J, Lai M, Li X, Goldberg DE, Beck JR, Zhou ZH. Ho CM, et al. Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2100514118. doi: 10.1073/pnas.2100514118. Proc Natl Acad Sci U S A. 2021. PMID: 34446549 Free PMC article. - Determination of Nitric Oxide and Its Metabolites in Biological Tissues Using Ozone-Based Chemiluminescence Detection: A State-of-the-Art Review.
Li J, LoBue A, Heuser SK, Cortese-Krott MM. Li J, et al. Antioxidants (Basel). 2024 Jan 31;13(2):179. doi: 10.3390/antiox13020179. Antioxidants (Basel). 2024. PMID: 38397777 Free PMC article. Review. - Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia.
Zhang R, Hess DT, Qian Z, Hausladen A, Fonseca F, Chaube R, Reynolds JD, Stamler JS. Zhang R, et al. Proc Natl Acad Sci U S A. 2015 May 19;112(20):6425-30. doi: 10.1073/pnas.1502285112. Epub 2015 Mar 25. Proc Natl Acad Sci U S A. 2015. PMID: 25810253 Free PMC article.
References
- Ross J.M., Fairchild H.M., Weldy J., Guyton A.C. Autoregulation of blood flow by oxygen lack. American Journal of Physiology. 1962;202:21–24. - PubMed
- Jia L., Bonaventura C., Bonaventura J., Stamler J.S. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. - PubMed
- Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X., Huang K.T., Shields H., Kim-Shapiro D.B., Schechter A.N., Cannon R.O., 3rd, Gladwin M.T. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9:1498–1505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous