Design of the Bracing in Adolescent Idiopathic Scoliosis Trial (BrAIST) - PubMed (original) (raw)
Randomized Controlled Trial
Design of the Bracing in Adolescent Idiopathic Scoliosis Trial (BrAIST)
Stuart L Weinstein et al. Spine (Phila Pa 1976). 2013.
Abstract
Study design: Descriptive.
Objective: To describe the design and development of Bracing in Adolescent Idiopathic Scoliosis Trial (BrAIST).
Summary of background data: Bracing has remained the standard of care for the nonoperative treatment of adolescent idiopathic scoliosis since the introduction of the Milwaukee brace in the late 1940s, but it has never been subjected to a rigorous evaluation of either its efficacy or its effectiveness. The BrAIST was designed to address the primary question: Do braces (specifically a thoracolumbosacral orthosis) lower the risk of curve progression to a surgical threshold (≥50°) in patients with adolescent idiopathic scoliosis relative to watchful waiting alone?
Methods: The authors describe the rationale for BrAIST, including the limitations of the current literature evaluating bracing for adolescent idiopathic scoliosis. Second, the authors describe the preliminary work, including the preparation of the National Institutes of Health clinical trials planning grant. Finally, the authors describe the trial design in detail.
Results: BrAIST was conducted in 25 sites in North America. Subjects were treated either with a thoracolumbosacral orthosis or watchful waiting and followed every 6 months until they reached skeletal maturity or the surgical threshold of 50° Cobb angle.
Conclusion: Clinical decision making will be improved by translation of the BrAIST results into evidence-based prognosis and estimates of how the prognosis, specifically the risk of progressing to surgery, may be altered by the use of bracing.
Level of evidence: N/A.
Figures
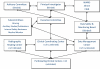
Figure 1
Organizational Structure of BrAIST
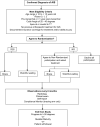
Figure 2
Subject Flow Chart
Similar articles
- Bracing in Adolescent Idiopathic Scoliosis Trial (BrAIST): Development and Validation of a Prognostic Model in Untreated Adolescent Idiopathic Scoliosis Using the Simplified Skeletal Maturity System.
Dolan LA, Weinstein SL, Abel MF, Bosch PP, Dobbs MB, Farber TO, Halsey MF, Hresko MT, Krengel WF, Mehlman CT, Sanders JO, Schwend RM, Shah SA, Verma K. Dolan LA, et al. Spine Deform. 2019 Nov;7(6):890-898.e4. doi: 10.1016/j.jspd.2019.01.011. Spine Deform. 2019. PMID: 31731999 Free PMC article. Clinical Trial. - Adolescent Idiopathic Scoliosis Bracing Success Is Influenced by Time in Brace: Comparative Effectiveness Analysis of BrAIST and ISICO Cohorts.
Dolan LA, Donzelli S, Zaina F, Weinstein SL, Negrini S. Dolan LA, et al. Spine (Phila Pa 1976). 2020 Sep 1;45(17):1193-1199. doi: 10.1097/BRS.0000000000003506. Spine (Phila Pa 1976). 2020. PMID: 32205704 Clinical Trial. - BrAIST-Calc: Prediction of Individualized Benefit From Bracing for Adolescent Idiopathic Scoliosis.
Dolan LA, Weinstein SL, Dobbs MB, Flynn JMJ, Green DW, Halsey MF, Hresko MT, Krengel WF 3rd, Mehlman CT, Milbrandt TA, Newton PO, Price N, Sanders JO, Schmitz ML, Schwend RM, Shah SA, Song K, Talwalkar V. Dolan LA, et al. Spine (Phila Pa 1976). 2024 Feb 1;49(3):147-156. doi: 10.1097/BRS.0000000000004879. Epub 2023 Nov 23. Spine (Phila Pa 1976). 2024. PMID: 37994691 - Braces for idiopathic scoliosis in adolescents.
Negrini S, Minozzi S, Bettany-Saltikov J, Chockalingam N, Grivas TB, Kotwicki T, Maruyama T, Romano M, Zaina F. Negrini S, et al. Cochrane Database Syst Rev. 2015 Jun 18;2015(6):CD006850. doi: 10.1002/14651858.CD006850.pub3. Cochrane Database Syst Rev. 2015. PMID: 26086959 Free PMC article. Review. - Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS Committee on Bracing and Nonoperative Management.
Richards BS, Bernstein RM, D'Amato CR, Thompson GH. Richards BS, et al. Spine (Phila Pa 1976). 2005 Sep 15;30(18):2068-75; discussion 2076-7. doi: 10.1097/01.brs.0000178819.90239.d0. Spine (Phila Pa 1976). 2005. PMID: 16166897 Review.
Cited by
- Nighttime Bracing or Exercise in Moderate-Grade Adolescent Idiopathic Scoliosis: A Randomized Clinical Trial.
Charalampidis A, Diarbakerli E, Dufvenberg M, Jalalpour K, Ohlin A, Ahl AA, Möller H, Abbott A, Gerdhem P; CONTRAIS Study Group. Charalampidis A, et al. JAMA Netw Open. 2024 Jan 2;7(1):e2352492. doi: 10.1001/jamanetworkopen.2023.52492. JAMA Netw Open. 2024. PMID: 38285447 Free PMC article. Clinical Trial. - Three-Dimensional Analysis of Initial Brace Correction in the Setting of Adolescent Idiopathic Scoliosis.
Almansour H, Pepke W, Bruckner T, Diebo BG, Akbar M. Almansour H, et al. J Clin Med. 2019 Oct 28;8(11):1804. doi: 10.3390/jcm8111804. J Clin Med. 2019. PMID: 31661811 Free PMC article. - Bracing in adolescent idiopathic scoliosis.
Schlenzka D, Yrjönen T. Schlenzka D, et al. J Child Orthop. 2013 Feb;7(1):51-5. doi: 10.1007/s11832-012-0464-5. Epub 2012 Nov 30. J Child Orthop. 2013. PMID: 24432059 Free PMC article. Review. - Long-term results after Boston brace treatment in late-onset juvenile and adolescent idiopathic scoliosis.
Lange JE, Steen H, Gunderson R, Brox JI. Lange JE, et al. Scoliosis. 2011 Aug 31;6:18. doi: 10.1186/1748-7161-6-18. Scoliosis. 2011. PMID: 21880123 Free PMC article. - Editorial on "Screening for adolescent idiopathic scoliosis: US preventive services task force recommendation statement".
Ha AS, Beauchamp EC. Ha AS, et al. J Spine Surg. 2018 Dec;4(4):812-816. doi: 10.21037/jss.2018.10.04. J Spine Surg. 2018. PMID: 30714016 Free PMC article. No abstract available.
References
- Nachemson AL, Lonstein JE, Weinstein SL. Scoliosis Research Society. Denver, Colorado: 1982. Report of the Prevalence and Natural History Committee of the Scoliosis Research Society.
- HCUP Kids’ Inpatient Database (KID) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2009. www.hcup-us.ahrq.gov/kidoverview.jsp.
- Pehrsson K, Larsson S, Oden A, et al. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine. 1992;17:1091–6. - PubMed
- Weinstein SL, Dolan LA, Spratt KF, et al. Health and function of patients with untreated idiopathic scoliosis: a 50-year natural history study. JAMA. 2003;289:559–67. - PubMed
- Ascani E, Bartolozzi P, Logroscino CA, et al. Natural history of untreated idiopathic scoliosis after skeletal maturity. Symposium on Epidemiology, Natural History and Non-operative Treatment of Idiopathic Scoliosis. Spine. 1986;11:784–9. - PubMed
Publication types
MeSH terms
Grants and funding
- R21AR049587/AR/NIAMS NIH HHS/United States
- R21 AR049587/AR/NIAMS NIH HHS/United States
- R01 AR052113/AR/NIAMS NIH HHS/United States
- FRN-81050/CAPMC/ CIHR/Canada
- R01AR052113/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials