Clinical and biological implications of driver mutations in myelodysplastic syndromes - PubMed (original) (raw)
. 2013 Nov 21;122(22):3616-27; quiz 3699.
doi: 10.1182/blood-2013-08-518886. Epub 2013 Sep 12.
Moritz Gerstung, Luca Malcovati, Sudhir Tauro, Gunes Gundem, Peter Van Loo, Chris J Yoon, Peter Ellis, David C Wedge, Andrea Pellagatti, Adam Shlien, Michael John Groves, Simon A Forbes, Keiran Raine, Jon Hinton, Laura J Mudie, Stuart McLaren, Claire Hardy, Calli Latimer, Matteo G Della Porta, Sarah O'Meara, Ilaria Ambaglio, Anna Galli, Adam P Butler, Gunilla Walldin, Jon W Teague, Lynn Quek, Alex Sternberg, Carlo Gambacorti-Passerini, Nicholas C P Cross, Anthony R Green, Jacqueline Boultwood, Paresh Vyas, Eva Hellstrom-Lindberg, David Bowen, Mario Cazzola, Michael R Stratton, Peter J Campbell; Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium
Affiliations
- PMID: 24030381
- PMCID: PMC3837510
- DOI: 10.1182/blood-2013-08-518886
Clinical and biological implications of driver mutations in myelodysplastic syndromes
Elli Papaemmanuil et al. Blood. 2013.
Abstract
Myelodysplastic syndromes (MDS) are a heterogeneous group of chronic hematological malignancies characterized by dysplasia, ineffective hematopoiesis and a variable risk of progression to acute myeloid leukemia. Sequencing of MDS genomes has identified mutations in genes implicated in RNA splicing, DNA modification, chromatin regulation, and cell signaling. We sequenced 111 genes across 738 patients with MDS or closely related neoplasms (including chronic myelomonocytic leukemia and MDS-myeloproliferative neoplasms) to explore the role of acquired mutations in MDS biology and clinical phenotype. Seventy-eight percent of patients had 1 or more oncogenic mutations. We identify complex patterns of pairwise association between genes, indicative of epistatic interactions involving components of the spliceosome machinery and epigenetic modifiers. Coupled with inferences on subclonal mutations, these data suggest a hypothesis of genetic "predestination," in which early driver mutations, typically affecting genes involved in RNA splicing, dictate future trajectories of disease evolution with distinct clinical phenotypes. Driver mutations had equivalent prognostic significance, whether clonal or subclonal, and leukemia-free survival deteriorated steadily as numbers of driver mutations increased. Thus, analysis of oncogenic mutations in large, well-characterized cohorts of patients illustrates the interconnections between the cancer genome and disease biology, with considerable potential for clinical application.
Figures
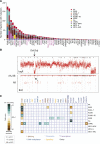
Figure 1
Genomic architecture of MDS. (A) Frequency of driver mutations identified in the sequencing screen or by cytogenetics in the cohort of 738 patients, broken down by MDS subtype. (B) Example of a copy number plot from a patient with a cytogenetically proven deletion on chromosome 5q. The upper panel depicts the normalized sequencing yields per exon; the lower panel depicts the variant allele fraction for germline SNPs. “AB” indicates the expected B-allele fractions for heterozygous SNPs; “AA” and “BB” indicate the position of the expected B-allele fractions for the homozygous SNPs AA and BB. (C) Associations among genes and cytogenetic abnormalities with disease subtypes in the study. Only associations with a q value (P value corrected for multiple hypothesis testing) <.1 are shown. Associations are colored by odds ratio. Blue-green colors depict gene-subtype associations that are observed together more than expected by chance, with brown colors depicting gene-subtype associations observed together less frequently than expected by chance.
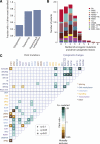
Figure 2
Oncogenic mutations identified in MDS. (A) Fraction of patients with at least 1 driver mutation, identified by cytogenetics, targeted gene sequencing, or sequencing combined with bone marrow cytogenetics. The fraction reported for targeted gene sequencing includes both oncogenic point mutations and copy number changes identified from the sequencing data alone. (B) Distribution of number of driver mutations (including point mutations, indels, and cytogenetic lesions) per patient broken down by MDS subtype. (C) Pairwise associations among genes and cytogenetic abnormalities found in at least 10 patients. Only associations with a q value (false discovery rate adjusted P value) <.1 are shown. Associations are colored by odds ratio. Brown colors depict mutually exclusive gene pairs (one or the other mutated, but rarely both together), and blue-green colors depict gene pairs that are comutated more than expected by chance. Gene names are color coded as per index on right side panel of the figure.
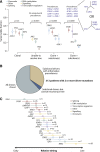
Figure 3
Clonal and subclonal driver mutations in MDS. (A) Variant allele fractions (y-axis) for driver mutations identified in 4 illustrative patients. The points show the observed allele fraction, with the vertical bars denoting 95% CIs in this fraction. The leftmost patient shows 4 driver mutations all at the same allele fraction. The second patient from the left shows statistical evidence for clonal heterogeneity, but the variant allele fractions are too low to establish phylogenetic relationships among mutations unambiguously. The rightmost 2 patients have statistically significant differences in observed allele fractions among driver mutations with some definitive phylogenetic structure. The phylogenetic tree cannot always be fully resolved (see possible trees for the 4th patient), but even with this uncertainty, 4 informative pairwise precedences can be unambiguously stated. (B) Pie chart showing the distribution of clonality and subclonality among 313 patients with 2 or more driver mutations. (C) Results of a Bradley-Terry model showing the relative temporal order of genes involved in at least 5 pairwise precedences. The estimates are calculated in relation to ASXL1 as the reference point, and standard errors are shown as horizontal bars. Genes are colored by their general biological function. A total of 107 patients contributed informative precedences.
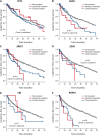
Figure 4
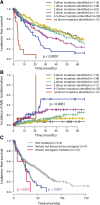
Outcome by whether driver mutations are clonal or subclonal. Leukemia-free survival for patients showing no mutation (gray), clonal driver mutations (blue), or subclonal driver mutations (red) for (A) TET2, (B) ASXL1, (C) SRSF2, (D) EZH2, (E) CBL, and (F) RUNX1. The P values denote the hypothesis test of whether splitting driver mutations into clonal or subclonal categories improves fit in a Cox proportional hazards model.
Figure 5
Relationship between number of oncogenic mutations and outcome. (A) Leukemia-free survival for patients broken down by how many oncogenic mutations were identified (including both point mutations and cytogenetic lesions). The mean number of cytogenetic lesions per patient was 0.2, 0.4, 0.5, 0.8, and 2.3 for patients with 1, 2, 3, 4 to 5, and 6 or more oncgenic mutations, respectively. The P value denotes the log-rank test of the null hypothesis that all groups had the same leukemia-free survival. (B) Incidence of transformation to acute leukemia broken down by how many oncogenic mutations were identified. (C) Leukemia-free survival for patients with no ASXL1 mutations (gray), “known oncogenic” mutations (blue), and “possible oncogenic” mutations or variants “of unknown significance” (red). The P values refer to log-rank tests comparing the class of mutation to those patients without ASXL1 mutations.
Figure 6
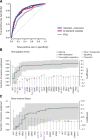
Predicting leukemia-free survival. (A) Receiver operating characteristic curves on cross-validation subsets for leukemia-free survival using 3 variable datasets: IPSS (gray); standard variable predictions made using all variables available from peripheral blood counts bone marrow evaluation, cytogenetics, and demographics (red); and sequencing in combination with all standard variables (blue). The further the curve deviates from the diagonal, the more informative the prognostic model is. (B) Multivariate model to predict hemoglobin levels from driver mutations. The green step curve shows the cumulative proportion of variance (left y-axis) in hemoglobin levels explained by each of the genetic variables as one proceeds from left to right along the x-axis. The gray shaded area represents the 95% CI for this curve. Coefficient estimates for each gene in the model including all variables (right y-axis) are shown as circles, colored by biological pathway and sized by the number of patients with the given lesion. Coefficients above 0 indicate positive correlation with hemoglobin levels. (C) Multivariate model to predict bone marrow blast count from driver mutations, as for panel B.
Comment in
- The importance of subclonal genetic events in MDS.
Bejar R, Abdel-Wahab O. Bejar R, et al. Blood. 2013 Nov 21;122(22):3550-1. doi: 10.1182/blood-2013-09-527655. Blood. 2013. PMID: 24263953
Similar articles
- Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia.
Malcovati L, Papaemmanuil E, Ambaglio I, Elena C, Gallì A, Della Porta MG, Travaglino E, Pietra D, Pascutto C, Ubezio M, Bono E, Da Vià MC, Brisci A, Bruno F, Cremonesi L, Ferrari M, Boveri E, Invernizzi R, Campbell PJ, Cazzola M. Malcovati L, et al. Blood. 2014 Aug 28;124(9):1513-21. doi: 10.1182/blood-2014-03-560227. Epub 2014 Jun 26. Blood. 2014. PMID: 24970933 Free PMC article. - Spliceosome mutations in myelodysplastic syndromes and chronic myelomonocytic leukemia.
Chesnais V, Kosmider O, Damm F, Itzykson R, Bernard OA, Solary E, Fontenay M. Chesnais V, et al. Oncotarget. 2012 Nov;3(11):1284-93. doi: 10.18632/oncotarget.749. Oncotarget. 2012. PMID: 23327988 Free PMC article. Review. - [Genomic aberrations in myelodysplastic syndromes and related disorders].
Makishima H. Makishima H. Rinsho Ketsueki. 2019;60(6):600-609. doi: 10.11406/rinketsu.60.600. Rinsho Ketsueki. 2019. PMID: 31281151 Review. Japanese. - Targeted Next-Generation Sequencing in Myelodysplastic Syndrome and Chronic Myelomonocytic Leukemia Aids Diagnosis in Challenging Cases and Identifies Frequent Spliceosome Mutations in Transformed Acute Myeloid Leukemia.
Reinig E, Yang F, Traer E, Arora R, Brown S, Rattray R, Braziel R, Fan G, Press R, Dunlap J. Reinig E, et al. Am J Clin Pathol. 2016 Apr;145(4):497-506. doi: 10.1093/ajcp/aqw016. Epub 2016 Apr 22. Am J Clin Pathol. 2016. PMID: 27124934 - Spliceosome mutations exhibit specific associations with epigenetic modifiers and proto-oncogenes mutated in myelodysplastic syndrome.
Mian SA, Smith AE, Kulasekararaj AG, Kizilors A, Mohamedali AM, Lea NC, Mitsopoulos K, Ford K, Nasser E, Seidl T, Mufti GJ. Mian SA, et al. Haematologica. 2013 Jul;98(7):1058-66. doi: 10.3324/haematol.2012.075325. Epub 2013 Jan 8. Haematologica. 2013. PMID: 23300180 Free PMC article.
Cited by
- Validation of WHO classification-based Prognostic Scoring System (WPSS) for myelodysplastic syndromes and comparison with the revised International Prognostic Scoring System (IPSS-R). A study of the International Working Group for Prognosis in Myelodysplasia (IWG-PM).
Della Porta MG, Tuechler H, Malcovati L, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SM, Miyazaki Y, Pfeilstöcker M, Sekeres MA, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D, Greenberg PL, Cazzola M. Della Porta MG, et al. Leukemia. 2015 Jul;29(7):1502-13. doi: 10.1038/leu.2015.55. Epub 2015 Feb 27. Leukemia. 2015. PMID: 25721895 - The altered transcriptome of pediatric myelodysplastic syndrome revealed by RNA sequencing.
Zubovic L, Piazza S, Tebaldi T, Cozzuto L, Palazzo G, Sidarovich V, De Sanctis V, Bertorelli R, Lammens T, Hofmans M, De Moerloose B, Ponomarenko J, Pigazzi M, Masetti R, Mecucci C, Basso G, Macchi P. Zubovic L, et al. J Hematol Oncol. 2020 Oct 12;13(1):135. doi: 10.1186/s13045-020-00974-3. J Hematol Oncol. 2020. PMID: 33046098 Free PMC article. - High prevalence and allele burden-independent prognostic importance of p53 mutations in an inner-city MDS/AML cohort.
Goel S, Hall J, Pradhan K, Hirsch C, Przychodzen B, Shastri A, Mantzaris I, Janakiram M, Battini R, Kornblum N, Derman O, Gritsman K, Al-Hafidh J, Wang Y, Halmos B, Steidl U, Maciejewski JP, Braunschweig I, Verma A. Goel S, et al. Leukemia. 2016 Aug;30(8):1793-5. doi: 10.1038/leu.2016.74. Epub 2016 Apr 29. Leukemia. 2016. PMID: 27125205 No abstract available. - Myelodysplastic syndromes, version 2.2015.
Greenberg PL, Stone RM, Bejar R, Bennett JM, Bloomfield CD, Borate U, De Castro CM, Deeg HJ, DeZern AE, Fathi AT, Frankfurt O, Gaensler K, Garcia-Manero G, Griffiths EA, Head D, Klimek V, Komrokji R, Kujawski LA, Maness LJ, O'Donnell MR, Pollyea DA, Scott B, Shami PJ, Stein BL, Westervelt P, Wheeler B, Shead DA, Smith C; National comprehensive cancer network. Greenberg PL, et al. J Natl Compr Canc Netw. 2015 Mar;13(3):261-72. doi: 10.6004/jnccn.2015.0038. J Natl Compr Canc Netw. 2015. PMID: 25736003 Free PMC article. - Clinical impacts of severe thrombocytopenia in the first cycle of azacitidine monotherapy and cytogenetics in patients with myelodysplastic syndrome: The Kyoto Conditional Survival Scoring System.
Inoue Y, Okamoto H, Miyashita A, Kawaji-Kanayama Y, Chinen S, Fujino T, Tsukamoto T, Shimura Y, Mizutani S, Kaneko H, Kuwahara-Ota S, Fuchida SI, Nishiyama D, Hirakawa K, Uchiyama H, Uoshima N, Kawata E, Kuroda J. Inoue Y, et al. Oncol Lett. 2023 Dec 18;27(2):62. doi: 10.3892/ol.2023.14193. eCollection 2024 Feb. Oncol Lett. 2023. PMID: 38192677 Free PMC article.
References
- Garraway LA. Genomics-driven oncology: framework for an emerging paradigm. J Clin Oncol. 2013;31(15):1806–1814. - PubMed
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872–1885. - PubMed
- Font P, Loscertales J, Benavente C, et al. Inter-observer variance with the diagnosis of myelodysplastic syndromes (MDS) following the 2008 WHO classification. Ann Hematol. 2013 92(1):19-24. - PubMed
Publication types
MeSH terms
Grants and funding
- 088340/WT_/Wellcome Trust/United Kingdom
- WT088340MA/WT_/Wellcome Trust/United Kingdom
- MC_U137961146/MRC_/Medical Research Council/United Kingdom
- 077012/Z/05/Z/WT_/Wellcome Trust/United Kingdom
- 100140/WT_/Wellcome Trust/United Kingdom
- CRUK_/Cancer Research UK/United Kingdom
- G1000729/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous