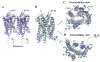Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex - PubMed (original) (raw)
. 2013 Sep 20;341(6152):1387-90.
doi: 10.1126/science.1241475. Epub 2013 Sep 12.
Ya Zhu, Jian Li, Zhuxi Chen, Gye Won Han, Irina Kufareva, Tingting Li, Limin Ma, Gustavo Fenalti, Jing Li, Wenru Zhang, Xin Xie, Huaiyu Yang, Hualiang Jiang, Vadim Cherezov, Hong Liu, Raymond C Stevens, Qiang Zhao, Beili Wu
Affiliations
- PMID: 24030490
- PMCID: PMC3819204
- DOI: 10.1126/science.1241475
Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex
Qiuxiang Tan et al. Science. 2013.
Abstract
The CCR5 chemokine receptor acts as a co-receptor for HIV-1 viral entry. Here we report the 2.7 angstrom-resolution crystal structure of human CCR5 bound to the marketed HIV drug maraviroc. The structure reveals a ligand-binding site that is distinct from the proposed major recognition sites for chemokines and the viral glycoprotein gp120, providing insights into the mechanism of allosteric inhibition of chemokine signaling and viral entry. A comparison between CCR5 and CXCR4 crystal structures, along with models of co-receptor-gp120-V3 complexes, suggests that different charge distributions and steric hindrances caused by residue substitutions may be major determinants of HIV-1 co-receptor selectivity. These high-resolution insights into CCR5 can enable structure-based drug discovery for the treatment of HIV-1 infection.
Figures
Fig. 1
Overall fold of the CCR5/Maraviroc complex and comparison with CXCR4. (A) Overall structure of the two CCR5-rubredoxin molecules related by pseudo-translational symmetry in one ASU. The receptor is colored blue, and the rubredoxin is light-cyan. The ligand Maraviroc is shown in orange sphere representation. The disulfide bonds are shown as yellow sticks. Zinc ions are shown as grey spheres. (B–D) Structure comparison between CCR5 (blue) and CXCR4 (PDB ID: 3ODU, green). The ligands are shown in stick representation. Maraviroc in CCR5 and IT1t in CXCR4 have orange and magenta carbons, respectively. C, top view of the extracellular side of CCR5 and CXCR4; D, bottom view of the intracellular side of CCR5 and CXCR4.
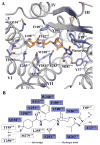
Fig. 2
CCR5 ligand binding pocket for Maraviroc. (A) Key residues in CCR5 for Maraviroc binding. Maraviroc (orange carbons) and receptor residues (blue carbons) involved in ligand binding are shown in stick representation. Other elements are colored as follows: oxygen, red; nitrogen, dark blue; sulfur, yellow; fluorine, light-cyan. The water molecule involved in interacting with Maraviroc is shown as a red sphere. (B) Schematic representation of interactions between CCR5 and Maraviroc. Mutations reported to be critical for Maraviroc binding are indicated with blue squares (11, 16).
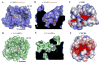
Fig. 3
Comparison of the ligand binding pockets between CCR5/Maraviroc and CXCR4/IT1t. (A, D) Top views of the ligand binding pockets in CCR5 (A, blue) and CXCR4 (D, green), showing a more open ligand binding pocket in CCR5. The receptors are shown in both cartoon and molecular surface representations. The ligands are shown in stick representation. Maraviroc in CCR5 and IT1t in CXCR4 have orange and magenta carbons, respectively. (B, E) Side views of the ligand binding pockets in CCR5 (B) and CXCR4 (E), showing that Maraviroc binds deeper in CCR5 than IT1t in CXCR4. (C, F) Top views of the ligand binding pockets in CCR5 (C) and CXCR4 (F). Both CCR5 and CXCR4 surfaces are colored according to their electrostatic potential from red (negative) to blue (positive), showing different charge distribution within the ligand binding pockets of these two receptors.
Comment in
- Structural biology. A new bundle of prospects for blocking HIV-1 entry.
Klasse PJ. Klasse PJ. Science. 2013 Sep 20;341(6152):1347-8. doi: 10.1126/science.1245384. Epub 2013 Sep 12. Science. 2013. PMID: 24030494 No abstract available.
Similar articles
- Structure of CC Chemokine Receptor 5 with a Potent Chemokine Antagonist Reveals Mechanisms of Chemokine Recognition and Molecular Mimicry by HIV.
Zheng Y, Han GW, Abagyan R, Wu B, Stevens RC, Cherezov V, Kufareva I, Handel TM. Zheng Y, et al. Immunity. 2017 Jun 20;46(6):1005-1017.e5. doi: 10.1016/j.immuni.2017.05.002. Immunity. 2017. PMID: 28636951 Free PMC article. - [Viral entry as therapeutic target. Current situation of entry inhibitors].
Arenzana-Seisdedos F. Arenzana-Seisdedos F. Enferm Infecc Microbiol Clin. 2008 Oct;26 Suppl 11:5-11. doi: 10.1016/s0213-005x(08)76557-1. Enferm Infecc Microbiol Clin. 2008. PMID: 19133215 Review. Spanish. - Characterizing the Diverse Mutational Pathways Associated with R5-Tropic Maraviroc Resistance: HIV-1 That Uses the Drug-Bound CCR5 Coreceptor.
Jiang X, Feyertag F, Meehan CJ, McCormack GP, Travers SA, Craig C, Westby M, Lewis M, Robertson DL. Jiang X, et al. J Virol. 2015 Nov;89(22):11457-72. doi: 10.1128/JVI.01384-15. Epub 2015 Sep 2. J Virol. 2015. PMID: 26339063 Free PMC article. - Molecular recognition of CCR5 by an HIV-1 gp120 V3 loop.
Tamamis P, Floudas CA. Tamamis P, et al. PLoS One. 2014 Apr 24;9(4):e95767. doi: 10.1371/journal.pone.0095767. eCollection 2014. PLoS One. 2014. PMID: 24763408 Free PMC article. - Effect of HIV-1 subtype and tropism on treatment with chemokine coreceptor entry inhibitors; overview of viral entry inhibition.
Panos G, Watson DC. Panos G, et al. Crit Rev Microbiol. 2015;41(4):473-87. doi: 10.3109/1040841X.2013.867829. Epub 2014 Mar 17. Crit Rev Microbiol. 2015. PMID: 24635642 Review.
Cited by
- Identification and interaction mechanism of novel small molecule antagonists targeting CC chemokine receptor 1/3/5 for treatment of non-small cell lung cancer.
Wang X, Wang J, Fu Q, Luo J, Shu M, Lin Z. Wang X, et al. Mol Divers. 2024 Nov 22. doi: 10.1007/s11030-024-11025-1. Online ahead of print. Mol Divers. 2024. PMID: 39578293 - Chemokinergic and Dopaminergic Signalling Collaborates through the Heteromer Formed by CCR9 and Dopamine Receptor D5 Increasing the Migratory Speed of Effector CD4+ T-Cells to Infiltrate the Colonic Mucosa.
Campos J, Osorio-Barrios F, Villanelo F, Gutierrez-Maldonado SE, Vargas P, Pérez-Acle T, Pacheco R. Campos J, et al. Int J Mol Sci. 2024 Sep 18;25(18):10022. doi: 10.3390/ijms251810022. Int J Mol Sci. 2024. PMID: 39337509 Free PMC article. - 7D, a small molecule inhibits dengue infection by increasing interferons and neutralizing-antibodies via CXCL4:CXCR3:p38:IRF3 and Sirt1:STAT3 axes respectively.
Gaur KK, Asuru TR, Srivastava M, Singh N, Purushotham N, Poojary B, Das B, Bhattacharyya S, Asthana S, Guchhait P. Gaur KK, et al. EMBO Mol Med. 2024 Oct;16(10):2376-2401. doi: 10.1038/s44321-024-00137-8. Epub 2024 Sep 16. EMBO Mol Med. 2024. PMID: 39284947 Free PMC article. - Exploring an Intracellular Allosteric Site of CC-Chemokine Receptor 4 from 3D Models, Probe Simulations, and Mutagenesis.
Ding T, Guseinov AA, Milligan G, Plouffe B, Tikhonova IG. Ding T, et al. ACS Pharmacol Transl Sci. 2024 Jul 16;7(8):2516-2526. doi: 10.1021/acsptsci.4c00330. eCollection 2024 Aug 9. ACS Pharmacol Transl Sci. 2024. PMID: 39144548 - Development of Anti-HIV Therapeutics: From Conventional Drug Discovery to Cutting-Edge Technology.
Sun Y, Wang L. Sun Y, et al. Pharmaceuticals (Basel). 2024 Jul 4;17(7):887. doi: 10.3390/ph17070887. Pharmaceuticals (Basel). 2024. PMID: 39065738 Free PMC article. Review.
References
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565. - PubMed
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108. - PubMed
- Blanpain C, et al. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood. 1999;94:1899. - PubMed
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI100604/AI/NIAID NIH HHS/United States
- R01 GM071872/GM/NIGMS NIH HHS/United States
- U01 GM094612/GM/NIGMS NIH HHS/United States
- U54 GM094618/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases