Protein crystallography for aspiring crystallographers or how to avoid pitfalls and traps in macromolecular structure determination - PubMed (original) (raw)
Review
. 2013 Nov;280(22):5705-36.
doi: 10.1111/febs.12495. Epub 2013 Sep 18.
Affiliations
- PMID: 24034303
- PMCID: PMC4080831
- DOI: 10.1111/febs.12495
Review
Protein crystallography for aspiring crystallographers or how to avoid pitfalls and traps in macromolecular structure determination
Alexander Wlodawer et al. FEBS J. 2013 Nov.
Abstract
The number of macromolecular structures deposited in the Protein Data Bank now approaches 100,000, with the vast majority of them determined by crystallographic methods. Thousands of papers describing such structures have been published in the scientific literature, and 20 Nobel Prizes in chemistry or medicine have been awarded for discoveries based on macromolecular crystallography. New hardware and software tools have made crystallography appear to be an almost routine (but still far from being analytical) technique and many structures are now being determined by scientists with very limited experience in the practical aspects of the field. However, this apparent ease is sometimes illusory and proper procedures need to be followed to maintain high standards of structure quality. In addition, many noncrystallographers may have problems with the critical evaluation and interpretation of structural results published in the scientific literature. The present review provides an outline of the technical aspects of crystallography for less experienced practitioners, as well as information that might be useful for users of macromolecular structures, aiming to show them how to interpret (but not overinterpret) the information present in the coordinate files and in their description. A discussion of the extent of information that can be gleaned from the atomic coordinates of structures solved at different resolution is provided, as well as problems and pitfalls encountered in structure determination and interpretation.
Keywords: data collection and processing; electron density maps; protein crystallography; structure quality; structure refinement; structure solution; structure validation.
© 2013 FEBS.
Figures
Fig. 1
A challenge: try to match the crystals with their diffraction patterns. Would you be able to match two out of the three crystals shown in (A), (B) and (C) with the X-ray diffraction patterns in (D) and (E)? The answer: the best diffraction pattern (D) was recorded for the ugliest specimen (C). The good looking crystal shown in (B) gave very poor diffraction (E), and the perfect looking crystals in (A) gave no diffraction at all (not shown). (A) Crystals of M. truncatula serine/threonine protein kinase. (B) Crystal of survivin B from X. laevis. (C) Crystal of a synthetic Z-DNA dodecamer. (D) Diffraction image taken from the top part of the crystal of Z-DNA dodecamer shown in (D). The data (to 0.75 Å resolution) were obtained with a PILATUS detector at the NE-CAT beamline of the Advanced Photon Source (Argonne National Laboratory, IL, USA). (E) Diffraction image of survivin B taken for the crystal shown in (B) with an ADSC Quantum315 detector at the SBCCAT beamline of the Advanced Photon Source. Only a few weak low-resolution reflections can be seen in the inset. The ring beyond the 4 Å mark is a result of ice and indicates problems with cryo-cooling of this crystal.
Fig. 2
The appearance of electron density as a function of the resolution of the experimental data. A cytosine–guanine pair from the structure of a Z-DNA hexamer duplex (PDB code:
3P4J
) [135] with the (_F_obs, αcalc) maps calculated with different resolution cut-offs at 0.55, 0.8, 1.2, 1,5, 2.0 and 3.0 Å. Although, at the highest resolution of 0.55 Å, there were 75 122 reflections included in map calculation, at 3 Å resolution, only 573 reflections were used.
Fig. 3
Electron density for regions with disorder. (A) The model and the corresponding (_F_obs, αcalc) map for statically disordered Lys87 in the structure of bovine trypsin (PDB code:
4I8G
) [136], with its side chain in two conformations. The map was calculated at 0.8 Å resolution and displayed at the 1.4σ contour level. (B) Lys178 from Erwinia chrysanthemi
l
-asparaginase (PDB code:
1O7J
) and the corresponding (_F_obs, αcalc) map at 1.0 Å resolution, with well-defined main chain atoms but a dynamically disordered end of the side chain having no interpretable electron density.
Fig. 4
An example of a phantom ligand in a protein structure refined at high resolution. Electron density and the atomic model are shown for a fragment of the cyclic form of BPTI refined at 1.0 Å resolution (PDB code:
1K6U
). A weighted _2mF_obs – _DF_calc map (blue) was contoured at 1.5σ, whereas the _mF_obs – _DF_calc map was contoured at ± 2.5σ (green positive, red negative). It is clear that the ethylene glycol molecule was placed completely incorrectly and that, most likely, only a single water molecule should have been modelled in its place.
Fig. 5
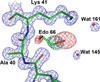
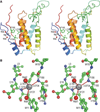
Chain tracing and selected details of the ‘enzyme’ frankensteinase. A few problems with this structure need to be emphasized. (1) No such protein has ever existed, nor is likely to exist in the future. (2) The coordinates were freely taken from several real proteins but were assembled by the creators with a significant dose of imagination. (3) An ‘active site’ consisting of the hydrophobic side chains of phenylalanine, leucine and valine is rather unlikely to have catalytic properties. (4) Identification of a metal ion that is not properly coordinated by any part of the protein is rather doubtful. (5) The distances between the ion and the ‘coordinating’ atoms are shown with the precision of four decimal digits, vastly exceeding their accuracy. Besides, the ‘bond’ distances and ‘coordination’ by amide N-H groups are entirely unacceptable for magnesium. This figure was taken directly from our previously published review [1]. (A) A stereoview showing a tracing of the protein chain in rainbow colours, changing from the blue N terminus to red C terminus. Active site residues are in ball-and-stick rendering, the Mg2+ ion is shown as a grey ball, and water molecules as red spheres. (B) A detail of the Mg2+ binding site, with carbon atoms coloured in green, oxygen in red and nitrogen in blue.
Fig. 6
Two examples of a Ramachandran plot. (A) A plot for Erwinia chrysanthemi
l
-asparaginase, one of the largest structures solved to date at atomic resolution (PDB code:
1O7J
), where one of the lysine residues (Lys178; labelled) has an unusual main-chain conformation that is, however, strongly supported by the electron density shown in Fig. 3B. (B) A plot for the fictitious ‘enzyme’ frankensteinase characterized by a very large number of main-chain dihedral angle violations found outside of the allowed regions. Unfortunately, many of these outliers originated from a part of a protein taken from a legitimate PDB entry, which should remain anonymous.
Similar articles
- Protein crystallography for non-crystallographers, or how to get the best (but not more) from published macromolecular structures.
Wlodawer A, Minor W, Dauter Z, Jaskolski M. Wlodawer A, et al. FEBS J. 2008 Jan;275(1):1-21. doi: 10.1111/j.1742-4658.2007.06178.x. Epub 2007 Nov 23. FEBS J. 2008. PMID: 18034855 Free PMC article. Review. - A Practical Approach to Protein Crystallography.
Ilari A, Savino C. Ilari A, et al. Methods Mol Biol. 2017;1525:47-78. doi: 10.1007/978-1-4939-6622-6_3. Methods Mol Biol. 2017. PMID: 27896717 - You are lost without a map: Navigating the sea of protein structures.
Lamb AL, Kappock TJ, Silvaggi NR. Lamb AL, et al. Biochim Biophys Acta. 2015 Apr;1854(4):258-68. doi: 10.1016/j.bbapap.2014.12.021. Epub 2014 Dec 29. Biochim Biophys Acta. 2015. PMID: 25554228 Free PMC article. Review. - Stereochemistry and Validation of Macromolecular Structures.
Wlodawer A. Wlodawer A. Methods Mol Biol. 2017;1607:595-610. doi: 10.1007/978-1-4939-7000-1_24. Methods Mol Biol. 2017. PMID: 28573590 Free PMC article. Review. - Super-resolution biomolecular crystallography with low-resolution data.
Schröder GF, Levitt M, Brunger AT. Schröder GF, et al. Nature. 2010 Apr 22;464(7292):1218-22. doi: 10.1038/nature08892. Epub 2010 Apr 7. Nature. 2010. PMID: 20376006 Free PMC article.
Cited by
- Atomic resolution structure of a protein prepared by non-enzymatic His-tag removal. Crystallographic and NMR study of GmSPI-2 inhibitor.
Kopera E, Bal W, Lenarčič Živkovič M, Dvornyk A, Kludkiewicz B, Grzelak K, Zhukov I, Zagórski-Ostoja W, Jaskolski M, Krzywda S. Kopera E, et al. PLoS One. 2014 Sep 18;9(9):e106936. doi: 10.1371/journal.pone.0106936. eCollection 2014. PLoS One. 2014. PMID: 25233114 Free PMC article. - Assigning crystallographic electron densities with free energy calculations-The case of the fluoride channel Fluc.
Ariz-Extreme I, Hub JS. Ariz-Extreme I, et al. PLoS One. 2018 May 17;13(5):e0196751. doi: 10.1371/journal.pone.0196751. eCollection 2018. PLoS One. 2018. PMID: 29771936 Free PMC article. - Combining X-Ray Crystallography with Small Angle X-Ray Scattering to Model Unstructured Regions of Nsa1 from S. Cerevisiae.
Lo YH, Pillon MC, Stanley RE. Lo YH, et al. J Vis Exp. 2018 Jan 10;(131):56953. doi: 10.3791/56953. J Vis Exp. 2018. PMID: 29364241 Free PMC article. - On the reliability of peptide nonplanarity seen in ultra-high resolution crystal structures.
Brereton AE, Karplus PA. Brereton AE, et al. Protein Sci. 2016 Apr;25(4):926-32. doi: 10.1002/pro.2883. Epub 2016 Feb 8. Protein Sci. 2016. PMID: 26779991 Free PMC article. - How to assess the structural dynamics of transcription factors by integrating sparse NMR and EPR constraints with molecular dynamics simulations.
Kozak F, Kurzbach D. Kozak F, et al. Comput Struct Biotechnol J. 2021 Apr 21;19:2097-2105. doi: 10.1016/j.csbj.2021.04.020. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 33995905 Free PMC article. Review.
References
- Bernstein FC, Koetzle TF, Williams GJB, Meyer EF, Jr, Brice MD, Rogers JR, Kennard O, Shimanouchi T, Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977;112:535–547. - PubMed
- Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature. 1958;181:662–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM053163/GM/NIGMS NIH HHS/United States
- GM094585/GM/NIGMS NIH HHS/United States
- U54 GM093342/GM/NIGMS NIH HHS/United States
- HHSN272201200026C/AI/NIAID NIH HHS/United States
- GM053163/GM/NIGMS NIH HHS/United States
- GM094662/GM/NIGMS NIH HHS/United States
- U54 GM094585/GM/NIGMS NIH HHS/United States
- ZIA BC010348-13/Intramural NIH HHS/United States
- GM093342/GM/NIGMS NIH HHS/United States
- U54 GM094662/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources