Virus budding and the ESCRT pathway - PubMed (original) (raw)
Review
Virus budding and the ESCRT pathway
Jörg Votteler et al. Cell Host Microbe. 2013.
Abstract
Enveloped viruses escape infected cells by budding through limiting membranes. In the decade since the discovery that HIV recruits cellular ESCRT (endosomal sorting complexes required for transport) machinery to facilitate viral budding, this pathway has emerged as the major escape route for enveloped viruses. In cells, the ESCRT pathway catalyzes analogous membrane fission events required for the abscission stage of cytokinesis and for a series of "reverse topology" vesiculation events. Studies of enveloped virus budding are therefore providing insights into the complex cellular mechanisms of cell division and membrane protein trafficking (and vice versa). Here, we review how viruses mimic cellular recruiting signals to usurp the ESCRT pathway, discuss mechanistic models for ESCRT pathway functions, and highlight important research frontiers.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
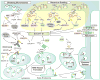
Figure 1. ESCRT pathway recruitment
Cellular and viral adaptor proteins and complexes that recruit early-acting ESCRT factors and NEDD4 family ubiquitin E3 ligases to different sites of ESCRT-dependent membrane fission are depicted schematically. The figure emphasizes how different retroviruses hijack the ESCRT pathway using late assembly domains within their Gag polyproteins (yellow background, Gag proteins in orange, with their constituent domains shown schematically), and how these interactions mimic analogous interactions between cellular adaptors (underlined) and ESCRT factors (white background). Polyubiquitin chains are depicted as connected yellow hexagons, solid arrows denote known protein-protein interactions, dashed arrows denote inferred or indirect protein-protein interactions, and question marks indicate that the site(s) of ubiquitin attachment are uncertain (see text). Abbreviations: HIV, Human Immunodeficiency Virus; RSV, Rous sarcoma virus; EIAV, Equine Infectious Anemia Virus; MVB, multivesicular body; ART, arrestin-related trafficking adaptor. For illustration purposes, we selected retroviruses that bud primarily through P(T/S)AP (HIV-1), PPXY (RSV) and YPXL (EIAV) late assembly domains, but note that these viruses all also use auxiliary late assembly domains, including an YPXL motif in RSV (not shown) (Dilley et al., 2010).
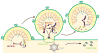
Figure 2. Multiple late assembly domains of HIV-1 Gag recruit different ESCRT-associated factors that may work together to facilitate virus budding
The model suggests how three different early-acting ESCRT-associated factors recruited by HIV-1 Gag -- NEDD4L (pink), ESCRT-I (red) and dimeric ALIX (dark blue) -- could work together in a stepwise fashion to facilitate virus budding. The three regions of HIV-1 Gag are depicted in yellow (MA), orange (CA) and red (NC, bound to blue RNA), the subunits of the trimeric viral Env protein are depicted in blue (SU/gp120) and pink (TM/gp41), and ESCRT-III proteins (light green) are depicted schematically as either polymeric filaments (central panel, light green ring) or soluble, autoinhibited subunits (right panel, discrete subunits). ESCRT-I is missing from the final panel because the ultimate fate of this complex is not yet clear.
Similar articles
- The regulation of Endosomal Sorting Complex Required for Transport and accessory proteins in multivesicular body sorting and enveloped viral budding - An overview.
Ahmed I, Akram Z, Iqbal HMN, Munn AL. Ahmed I, et al. Int J Biol Macromol. 2019 Apr 15;127:1-11. doi: 10.1016/j.ijbiomac.2019.01.015. Epub 2019 Jan 4. Int J Biol Macromol. 2019. PMID: 30615963 Review. - Hepatitis C Virus Proteins Interact with the Endosomal Sorting Complex Required for Transport (ESCRT) Machinery via Ubiquitination To Facilitate Viral Envelopment.
Barouch-Bentov R, Neveu G, Xiao F, Beer M, Bekerman E, Schor S, Campbell J, Boonyaratanakornkit J, Lindenbach B, Lu A, Jacob Y, Einav S. Barouch-Bentov R, et al. mBio. 2016 Nov 1;7(6):e01456-16. doi: 10.1128/mBio.01456-16. mBio. 2016. PMID: 27803188 Free PMC article. - The Interplay between ESCRT and Viral Factors in the Enveloped Virus Life Cycle.
Meng B, Lever AML. Meng B, et al. Viruses. 2021 Feb 20;13(2):324. doi: 10.3390/v13020324. Viruses. 2021. PMID: 33672541 Free PMC article. Review. - ESCRT machinery and cytokinesis: the road to daughter cell separation.
Caballe A, Martin-Serrano J. Caballe A, et al. Traffic. 2011 Oct;12(10):1318-26. doi: 10.1111/j.1600-0854.2011.01244.x. Epub 2011 Jul 27. Traffic. 2011. PMID: 21722282 Review. - The Role of Exosome and the ESCRT Pathway on Enveloped Virus Infection.
Ju Y, Bai H, Ren L, Zhang L. Ju Y, et al. Int J Mol Sci. 2021 Aug 22;22(16):9060. doi: 10.3390/ijms22169060. Int J Mol Sci. 2021. PMID: 34445766 Free PMC article. Review.
Cited by
- Prazoles Targeting Tsg101 Inhibit Release of Epstein-Barr Virus following Reactivation from Latency.
Mannemuddhu SS, Xu H, Bleck CKE, Tjandra N, Carter C, Bhaduri-McIntosh S. Mannemuddhu SS, et al. J Virol. 2021 Jun 10;95(13):e0246620. doi: 10.1128/JVI.02466-20. Epub 2021 Jun 10. J Virol. 2021. PMID: 33853959 Free PMC article. - Vps4 disassembles an ESCRT-III filament by global unfolding and processive translocation.
Yang B, Stjepanovic G, Shen Q, Martin A, Hurley JH. Yang B, et al. Nat Struct Mol Biol. 2015 Jun;22(6):492-8. doi: 10.1038/nsmb.3015. Epub 2015 May 4. Nat Struct Mol Biol. 2015. PMID: 25938660 Free PMC article. - Apoptosis and Phagocytosis as Antiviral Mechanisms.
Nainu F, Ophinni Y, Shiratsuchi A, Nakanishi Y. Nainu F, et al. Subcell Biochem. 2023;106:77-112. doi: 10.1007/978-3-031-40086-5_3. Subcell Biochem. 2023. PMID: 38159224 - Multiple Roles of the Cytoplasmic Domain of Herpes Simplex Virus 1 Envelope Glycoprotein D in Infected Cells.
Arii J, Shindo K, Koyanagi N, Kato A, Kawaguchi Y. Arii J, et al. J Virol. 2016 Oct 28;90(22):10170-10181. doi: 10.1128/JVI.01396-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27581980 Free PMC article. - The ESCRT and autophagy machineries cooperate to repair ESX-1-dependent damage at the Mycobacterium-containing vacuole but have opposite impact on containing the infection.
López-Jiménez AT, Cardenal-Muñoz E, Leuba F, Gerstenmaier L, Barisch C, Hagedorn M, King JS, Soldati T. López-Jiménez AT, et al. PLoS Pathog. 2018 Dec 31;14(12):e1007501. doi: 10.1371/journal.ppat.1007501. eCollection 2018 Dec. PLoS Pathog. 2018. PMID: 30596802 Free PMC article.
References
- Agromayor M, Martin-Serrano J. Knowing when to cut and run: mechanisms that control cytokinetic abscission. Trends Cell Biol. 2013 Epublication ahead of print. - PubMed
- Babst M, Katzmann D, Estepa-Sabal E, Meerloo T, Emr S. Escrt-III. An endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002;3:271–282. - PubMed
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. - PubMed
- Baumgartel V, Ivanchenko S, Dupont A, Sergeev M, Wiseman PW, Krausslich HG, Brauchle C, Muller B, Lamb DC. Live-cell visualization of dynamics of HIV budding site interactions with an ESCRT component. Nat Cell Biol. 2011;13:469–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI051174/AI/NIAID NIH HHS/United States
- GM082545/GM/NIGMS NIH HHS/United States
- R01 AI051174/AI/NIAID NIH HHS/United States
- R37 AI051174/AI/NIAID NIH HHS/United States
- P50 GM082545/GM/NIGMS NIH HHS/United States
- P01 GM066521/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources