A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition - PubMed (original) (raw)
. 2013 Oct;19(10):1331-1337.
doi: 10.1038/nm.3295. Epub 2013 Sep 15.
Stephanie M Piecewicz # 1, Lisa M McGinnis # 1, Cullen M Taniguchi 2, Stanley J Wiegand 3, Keith Anderson 3, Carol W-M Chan 1, Kimberly X Mulligan 4, David Kuo 1, Jenny Yuan 1, Mario Vallon 1, Lori Morton 3, Etienne Lefai 5, M Celeste Simon 6, Jacquelyn J Maher 7, Gilles Mithieux 8, Fabienne Rajas 8, Justin Annes 9, Owen P McGuinness 4, Gavin Thurston 3, Amato J Giaccia 2, Calvin J Kuo 1
Affiliations
- PMID: 24037094
- PMCID: PMC3795838
- DOI: 10.1038/nm.3295
A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition
Kevin Wei et al. Nat Med. 2013 Oct.
Abstract
Insulin initiates diverse hepatic metabolic responses, including gluconeogenic suppression and induction of glycogen synthesis and lipogenesis. The liver possesses a rich sinusoidal capillary network with a higher degree of hypoxia and lower gluconeogenesis in the perivenous zone as compared to the rest of the organ. Here, we show that diverse vascular endothelial growth factor (VEGF) inhibitors improved glucose tolerance in nondiabetic C57BL/6 and diabetic db/db mice, potentiating hepatic insulin signaling with lower gluconeogenic gene expression, higher glycogen storage and suppressed hepatic glucose production. VEGF inhibition induced hepatic hypoxia through sinusoidal vascular regression and sensitized liver insulin signaling through hypoxia-inducible factor-2α (Hif-2α, encoded by Epas1) stabilization. Notably, liver-specific constitutive activation of HIF-2α, but not HIF-1α, was sufficient to augment hepatic insulin signaling through direct and indirect induction of insulin receptor substrate-2 (Irs2), an essential insulin receptor adaptor protein. Further, liver Irs2 was both necessary and sufficient to mediate Hif-2α and Vegf inhibition effects on glucose tolerance and hepatic insulin signaling. These results demonstrate an unsuspected intersection between Hif-2α-mediated hypoxic signaling and hepatic insulin action through Irs2 induction, which can be co-opted by Vegf inhibitors to modulate glucose metabolism. These studies also indicate distinct roles in hepatic metabolism for Hif-1α, which promotes glycolysis, and Hif-2α, which suppresses gluconeogenesis, and suggest new treatment approaches for type 2 diabetes mellitus.
Figures
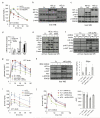
Figure 1. VEGF inhibition improves hepatic insulin action
a–d. Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) in adult C57Bl/6J (a) or db/db mice (b) treated with a single i.v. injection of Ad sFlt1/sVEGFR1, Ad sFlk1/sVEGFR2 or Ad Fc (n=8 each, 109 pfu) after 15 days. The initial average blood glucose levels for ITT in a. were Fc=129, sFlt1=72.8 and in b. were Fc=162, sFlt1=55.4, sFlk1=109, all mg/dL. c,d. GTT of adult SCID mice (n=5) or db/db mice (n=5) treated with aflibercept or hFc after 15 days. e. Hyperinsulinemic euglycemic clamp analysis of aflibercept- and hFc-treated db/db mice after 2 weeks. f. ELISA determination of fasting plasma insulin concentration from mice as in d. g,h. Insulin signaling pathway determination in fasted liver extracts after 14 days. i,j. Analysis of G6pc and Pepck mRNA by qRT-PCR from liver from Ad Fc, Ad sFlt1 and Ad sFlk1-injected mice (n=5, ad lib, day 14) (i) or adult SCID mice treated with aflibercept, (n=5, fasted, day 14) (j). Values are expressed as mean ± s.e.m. * = P<0.05.
Figure 2. VEGF inhibition induces hepatic vascular regression, liver hypoxia and HIF-2α stabilization to augment hepatic insulin signaling
a, b. Liver CD31 immunofluorescence and vessel area density percentage following Ad Fc, Ad sFlt1, Ad sFlk1, or aflibercept treatment after 14 days. Scale bar = 100 μm. c. FACS determination of hypoxia in mouse hepatocytes 14 days after Ad sFlt1-, Ad Flk1- (blue curves) or control Ad Fc- (red curves) treatment. Hypoxyprobe injection in mice was followed by anti-hydroxyprobe FITC-conjugated secondary antibody in disaggregated hepatocytes. The gray curve indicates a control liver without hypoxyprobe injection. d. Streptavidin-HRP staining of biotinylated L. esculentum lectin liver perfusion 22 days after Ad Fc, Ad sFlt1 and aflibercept treatment. Quantification of perfusion area density percentage is shown (n=3). e. HIF-2α and HIF-1α liver nuclear protein levels after 5 days Ad Fc and Ad sFlt1 treatment. f. GTT in 8–10 week old WT (Hif2αflox/flox) or HIF2LKO (Hif2αflox/flox; albumin-Cre) mice (n=6) 14 days after i.v. injection with Ad sFlt1 or Ad Fc. g. Liver Western blot from mice treated as in f. after 16 h fast. h. Densitometric quantitation of g. and G6pc liver qRT-PCR from f. Values are mean ± s.e.m. * = P<0.05.
Figure 3. Activation of hepatic HIF-2α but not HIF-1α signaling is sufficient to improve liver insulin action
a. GTT of C57Bl/6 mice 3d after single i.v. injection of Ad Fc, Ad Hif1αΔODD or Ad Hif2αPN. b, c. Liver extracts from animals in a. were analyzed by Western blot for IRS proteins (b) or for insulin signaling intermediates (c), 5d, ad lib. d. qPCR analysis of liver gluconeogenic gene expression, 5d, fasted. e. Western blot of hepatocytes 24 hours after Ad Fc, Ad Hif1αΔODD or Ad Hif2αPN infection, with or without 100 nM insulin stimulation. f. Western blot of primary mouse hepatocytes infected with Ad Fc or Ad Hif2αPN +/− Ad shRNA luc or Ad shRNA Irs2 for 24h, with and without 100 nM insulin stimulation. Short and long exposures for p-AKT are shown. g. GTT from db/db mice (n=8/group) 4 days after Ad Hif2αPN or Ad Fc treatment ± either Ad shRNA luciferase (shLuc) or Ad shRNA Irs2 (shIRS2). h. Western blot and qRT-PCR analysis of G6pc mRNA from liver of animals in g., ad lib. i. GTT from 8–10 week db/db mice treated with Ad Fc, Ad sFlt1, Ad Hif2αPN or Ad Irs2 after 5 days. j. GTT and AUC from C57Bl/6 mice at 5 days after injection with Ad Fc or Ad sFlt1 ± Ad shRNA luc or Ad shRNA Irs2 (n=6). Values are mean ± s.e.m. * = P<0.05.
Figure 4. HIF-2α regulates IRS2 through Srebp1c-dependent and –independent mechanisms
a. qRT-PCR analysis of Irs1 and Irs2 in C57lBl/6 mouse liver 5 days after Ad Hif2αPN or Hif1αΔODD treatment. b. qRT-PCR analysis of IRS2 in primary hepatocytes 24h post-infection with the indicated adenoviruses. c. Luciferase reporter assay in mouse H2 hepatocytes with human IRS2 promoter-luciferase constructs containing wild-type sequence (wt) or mutations of distal (−900mut) or proximal (−123mut) HREs with Ad GFP, Ad Hif2αΔODD or Ad Hif1αΔODD. d. Srebp1c qRT-PCR from primary hepatocytes infected with Ad Fc or Ad Hif2αPN ± insulin. e. Mouse liver Srebp1c qRT-PCR 5 days after Ad Fc, Ad Hif1αΔODD or Ad Hif2αPN treatment. f. Western blot of primary hepatocytes infected with either Ad Fc or Ad Hif2αPN ± Ad nSrebp-1c. g. IRS2 and nSREPB1c liver Western blot after Ad Fc or Ad Hif2αPN treatment ± Ad nSrebp1c. h. Model. HIF-2α signaling positively regulates the insulin receptor signaling pathway in hepatocytes through induction of IRS2 expression. HIF-2α, but not HIF-1α regulates IRS2 expression directly by promoter trans-activation; both HIF-2α and HIF-1α suppress Srebp1c which can facilitate HIF2α transactivation of IRS2. Physiologic liver hypoxia and VEGF inhibition via vascular regression represent two stimuli capable of activating the HIF2α→IRS2 pathway, possibly mediated by PHD3 (see accompanying manuscript by Taniguchi et al.). Values are expressed as mean ± s.e.m. * = P<0.05.
Similar articles
- Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes.
Taniguchi CM, Finger EC, Krieg AJ, Wu C, Diep AN, LaGory EL, Wei K, McGinnis LM, Yuan J, Kuo CJ, Giaccia AJ. Taniguchi CM, et al. Nat Med. 2013 Oct;19(10):1325-30. doi: 10.1038/nm.3294. Epub 2013 Sep 15. Nat Med. 2013. PMID: 24037093 Free PMC article. - Inhibition of prolyl hydroxylases increases hepatic insulin and decreases glucagon sensitivity by an HIF-2α-dependent mechanism.
Riopel M, Moon JS, Bandyopadhyay GK, You S, Lam K, Liu X, Kisseleva T, Brenner D, Lee YS. Riopel M, et al. Mol Metab. 2020 Nov;41:101039. doi: 10.1016/j.molmet.2020.101039. Epub 2020 Jun 11. Mol Metab. 2020. PMID: 32534258 Free PMC article. - A central role for hypoxia-inducible factor (HIF)-2α in hepatic glucose homeostasis.
Ramakrishnan SK, Shah YM. Ramakrishnan SK, et al. Nutr Healthy Aging. 2017 Dec 7;4(3):207-216. doi: 10.3233/NHA-170022. Nutr Healthy Aging. 2017. PMID: 29276790 Free PMC article. Review. - Mechanisms Leading to Differential Hypoxia-Inducible Factor Signaling in the Diabetic Kidney: Modulation by SGLT2 Inhibitors and Hypoxia Mimetics.
Packer M. Packer M. Am J Kidney Dis. 2021 Feb;77(2):280-286. doi: 10.1053/j.ajkd.2020.04.016. Epub 2020 Jul 23. Am J Kidney Dis. 2021. PMID: 32711072 Review.
Cited by
- Novel Genes Critical for Hypoxic Preconditioning in Zebrafish Are Regulators of Insulin and Glucose Metabolism.
Manchenkov T, Pasillas MP, Haddad GG, Imam FB. Manchenkov T, et al. G3 (Bethesda). 2015 Apr 3;5(6):1107-16. doi: 10.1534/g3.115.018010. G3 (Bethesda). 2015. PMID: 25840431 Free PMC article. - Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): clinical significance and therapeutic strategies.
Chan SMH, Selemidis S, Bozinovski S, Vlahos R. Chan SMH, et al. Pharmacol Ther. 2019 Jun;198:160-188. doi: 10.1016/j.pharmthera.2019.02.013. Epub 2019 Feb 26. Pharmacol Ther. 2019. PMID: 30822464 Free PMC article. Review. - Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer.
Jeong SH, Kim HB, Kim MC, Lee JM, Lee JH, Kim JH, Kim JW, Park WY, Kim SY, Kim JB, Kim H, Kim JM, Choi HS, Lim DS. Jeong SH, et al. J Clin Invest. 2018 Mar 1;128(3):1010-1025. doi: 10.1172/JCI95802. Epub 2018 Feb 5. J Clin Invest. 2018. PMID: 29400692 Free PMC article. - Regulation of erythroid differentiation in K562 cells by the EPAS1-IRS2 axis under hypoxic conditions.
Gao Z, Li Z, Li X, Xiao J, Li C. Gao Z, et al. Front Cell Dev Biol. 2023 Jun 1;11:1161541. doi: 10.3389/fcell.2023.1161541. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37325570 Free PMC article. - Transcriptome analysis of severe hypoxic stress during development in zebrafish.
Woods IG, Imam FB. Woods IG, et al. Genom Data. 2015 Aug 4;6:83-8. doi: 10.1016/j.gdata.2015.07.025. eCollection 2015 Dec. Genom Data. 2015. PMID: 26697342 Free PMC article.
References
- Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol. 2011;46:200–215. - PubMed
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. - PubMed
- Jungermann K. Zonation of metabolism and gene expression in liver. Histochem Cell Biol. 1995;103:81–91. - PubMed
- Kubota N, et al. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab. 2008;8:49–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DK043748/DK/NIDDK NIH HHS/United States
- T32 GM007365/GM/NIGMS NIH HHS/United States
- U24DK059637/DK/NIDDK NIH HHS/United States
- P30 DK026743/DK/NIDDK NIH HHS/United States
- CA67166/CA/NCI NIH HHS/United States
- R01HL074267/HL/NHLBI NIH HHS/United States
- T32 HL098049/HL/NHLBI NIH HHS/United States
- R01 HL074267/HL/NHLBI NIH HHS/United States
- GM-07365/GM/NIGMS NIH HHS/United States
- 1R01HL074267/HL/NHLBI NIH HHS/United States
- R01 CA158528/CA/NCI NIH HHS/United States
- R01CA158528/CA/NCI NIH HHS/United States
- K12 HL087746/HL/NHLBI NIH HHS/United States
- T32 DK007563/DK/NIDDK NIH HHS/United States
- 1K12HL087746/HL/NHLBI NIH HHS/United States
- T32DK007563/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- T32 AI007290/AI/NIAID NIH HHS/United States
- P01 CA067166/CA/NCI NIH HHS/United States
- 5T32AI07290/AI/NIAID NIH HHS/United States
- R01 DK043748/DK/NIDDK NIH HHS/United States
- R01 NS064517/NS/NINDS NIH HHS/United States
- DK084206/DK/NIDDK NIH HHS/United States
- R01NS064517/NS/NINDS NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous