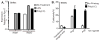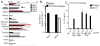Cutting edge: Mouse NAIP1 detects the type III secretion system needle protein - PubMed (original) (raw)
Cutting edge: Mouse NAIP1 detects the type III secretion system needle protein
Manira Rayamajhi et al. J Immunol. 2013.
Abstract
The NAIP/NLRC4 inflammasomes activate caspase-1 in response to bacterial type III secretion systems (T3SSs). Inadvertent injection of the T3SS rod protein and flagellin into the cytosol is detected through murine NAIP2 and NAIP5/6, respectively. In this study, we identify the agonist for the orphan murine NAIP1 receptor as the T3SS needle protein. NAIP1 is poorly expressed in resting mouse bone marrow-derived macrophages; however, priming with polyinosinic-polycytidylic acid induces it and confers needle protein sensitivity. Further, overexpression of NAIP1 in immortalized bone marrow-derived macrophages by retroviral transduction enabled needle detection. In contrast, peritoneal cavity macrophages basally express NAIP1 and respond to needle protein robustly, independent of priming. Human macrophages are known to express only one NAIP gene, which detects the needle protein, but not rod or flagellin. Thus, murine NAIP1 is functionally analogous to human NAIP.
Conflict of interest statement
Disclosures
The authors declare that they have no competing financial interests.
Figures
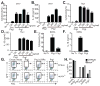
Figure 1. Human macrophages detect cytosolic PrgI while mouse macrophages show a weak response
Human monocytic cell lines U937 (A, B) and Thp1 (C, D) were differentiated into macrophages with phorbol myristate acetate (PMA), primed with 50 ng/ml LPS, and then transfected with purified PrgI or PrgJ. 2 h post transfection, cytotoxicity (A, C) and IL-1β secretion (B, D) were determined by LDH release and ELISA, respectively. BMMs primed with LPS (50ng/ml) for 4 hours were transfected with 125ng/well PrgI or PrgJ for 1 h and (E) cytotoxicity or (F) IL-1β secretion were determined. (G, H) Retroviral lethality screen was performed by transducing C57BL/6 and _Nlrc4_−/− BMMs with retroviruses expressing GFP alone (empty vector), _prgJ-_GFP, _prgJ_Δ_LRRs-_GFP, or _prgI_-GFP. Survival of GFP+ cells was determined by flow cytometry 2 days later by flow cytometry. Part of data in G and H (GFP alone and _prgJ_-GFP) were previously published in (8); _prgJ_Δ_LRRs_-GFP and prgI-GFP transduction data from the same experiment is now presented here. (H) Quantification of G. Graphs show mean and standard deviation of triplicate wells and are representative of at least 3 independent experiments.
Figure 2. Naip1 expression in BMMs with or without stimulation with LPS or PolyIC from the innate immune database
(A) BMMs were left untreated or primed with LPS or poly(I:C) 23 h and RQ-PCR was performed to assess Naip1 and Naip2 transcript levels relative to Rps17. (B) Cytotoxicity in response to cytosolic PrgI or PrgJ protein was determined at 2 h in unstimulated BMMs or BMMs primed with 6μg/ml Poly(I:C) for 2 days. Data representative of 2–3 independent experiments.
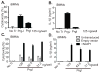
Figure 3. NAIP1 complementation confers robust PrgI sensitivity to BMMs
PrgI or PrgJ were transfected into the cytosol of iBMMs and (A) cytotoxicity and (B) IL-1β secretion were measured at 2 h. (C–D) iBMMs were transduced with retroviruses expressing either GFP alone (Empty vector) or _Naip1-_IRES-GFP and GFP+ cells were purified by FACS. (C) Cytotoxicity and (D) IL-1β secretion were determined 2 h after PrgI transfection. Data in (A–B) and (C–D) are representative of 2 and 3 independent experiments respectively.
Figure 4. Peritoneal cavity macrophages that express NAIP1 basally respond to T3SS needle
(A) Quantitative PCR was performed to assess the amounts of Naip1 (red bars), Naip2 (black bars), Naip5 (grey bars) and Naip6 (white bars) transcripts expressed in BMMs or the indicated organs and normalized to GAPDH. (B) Levels of Naip1 and Naip2 transcripts were determined in BMMs (white bars) or peritoneal cavity macrophages (PerC) by quantitative PCR.(C) PerC macrophages from C57BL/6 (black bars) or _Nlrc4_−/− mice (white bars) were primed with LPS (50ng/ml) before transfection with PrgI, PrgJ, or FlgE. IL-1β in the supernatants was measured at 2 h. Data representative of at least 2–3 independent observations.
Similar articles
- Broad detection of bacterial type III secretion system and flagellin proteins by the human NAIP/NLRC4 inflammasome.
Reyes Ruiz VM, Ramirez J, Naseer N, Palacio NM, Siddarthan IJ, Yan BM, Boyer MA, Pensinger DA, Sauer JD, Shin S. Reyes Ruiz VM, et al. Proc Natl Acad Sci U S A. 2017 Dec 12;114(50):13242-13247. doi: 10.1073/pnas.1710433114. Epub 2017 Nov 27. Proc Natl Acad Sci U S A. 2017. PMID: 29180436 Free PMC article. - Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation.
Yang J, Zhao Y, Shi J, Shao F. Yang J, et al. Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14408-13. doi: 10.1073/pnas.1306376110. Epub 2013 Aug 12. Proc Natl Acad Sci U S A. 2013. PMID: 23940371 Free PMC article. - The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus.
Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. Zhao Y, et al. Nature. 2011 Sep 14;477(7366):596-600. doi: 10.1038/nature10510. Nature. 2011. PMID: 21918512 - Emerging Concepts about NAIP/NLRC4 Inflammasomes.
Lage SL, Longo C, Branco LM, da Costa TB, Buzzo Cde L, Bortoluci KR. Lage SL, et al. Front Immunol. 2014 Jul 2;5:309. doi: 10.3389/fimmu.2014.00309. eCollection 2014. Front Immunol. 2014. PMID: 25071770 Free PMC article. Review. - The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus.
Zhao Y, Shao F. Zhao Y, et al. Immunol Rev. 2015 May;265(1):85-102. doi: 10.1111/imr.12293. Immunol Rev. 2015. PMID: 25879286 Review.
Cited by
- Robust microbe immune recognition in the intestinal mucosa.
Schären OP, Hapfelmeier S. Schären OP, et al. Genes Immun. 2021 Oct;22(5-6):268-275. doi: 10.1038/s41435-021-00131-x. Epub 2021 May 6. Genes Immun. 2021. PMID: 33958733 Free PMC article. Review. - Evasion and interference: intracellular pathogens modulate caspase-dependent inflammatory responses.
Stewart MK, Cookson BT. Stewart MK, et al. Nat Rev Microbiol. 2016 Jun;14(6):346-59. doi: 10.1038/nrmicro.2016.50. Epub 2016 May 13. Nat Rev Microbiol. 2016. PMID: 27174147 Review. - Inflammasomes: Threat-Assessment Organelles of the Innate Immune System.
Evavold CL, Kagan JC. Evavold CL, et al. Immunity. 2019 Oct 15;51(4):609-624. doi: 10.1016/j.immuni.2019.08.005. Epub 2019 Aug 28. Immunity. 2019. PMID: 31473100 Free PMC article. Review. - Yersinia Type III-Secreted Effectors Evade the Caspase-4 Inflammasome in Human Cells.
Zhang J, Brodsky IE, Shin S. Zhang J, et al. bioRxiv [Preprint]. 2023 Jun 16:2023.01.24.525473. doi: 10.1101/2023.01.24.525473. bioRxiv. 2023. PMID: 36747770 Free PMC article. Updated. Preprint. - Cryo-EM studies of NAIP-NLRC4 inflammasomes.
Haloupek N, Grob P, Tenthorey J, Vance RE, Nogales E. Haloupek N, et al. Methods Enzymol. 2019;625:177-204. doi: 10.1016/bs.mie.2019.04.030. Epub 2019 May 24. Methods Enzymol. 2019. PMID: 31455527 Free PMC article.
References
- Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Özören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Núñez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. - PubMed
- Sun YH, Rolan HG, Tsolis RM. Injection of Flagellin into the Host Cell Cytosol by Salmonella enterica Serotype Typhimurium. Journal of Biological Chemistry. 2007;282:33897–33901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases