The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis - PubMed (original) (raw)
. 2013 Jun 4;2(3):227-42.
doi: 10.1016/j.molmet.2013.05.006. eCollection 2013.
Michael D Neinast, Kai Sun, William M Skiles, Jessica D Bills, Jordan A Zehr, Daniel Zeve, Lisa D Hahner, Derek W Cox, Lana M Gent, Yong Xu, Zhao V Wang, Sohaib A Khan, Deborah J Clegg
Affiliations
- PMID: 24049737
- PMCID: PMC3773827
- DOI: 10.1016/j.molmet.2013.05.006
The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis
Kathryn E Davis et al. Mol Metab. 2013.
Abstract
Our data demonstrate that estrogens, estrogen receptor-α (ERα), and estrogen receptor-β (ERβ) regulate adipose tissue distribution, inflammation, fibrosis, and glucose homeostasis, by determining that αERKO mice have increased adipose tissue inflammation and fibrosis prior to obesity onset. Selective deletion of adipose tissue ERα in adult mice using a novel viral vector technology recapitulated the findings in the total body ERα null mice. Generation of a novel mouse model, lacking ERα specifically from adipocytes (AdipoERα), demonstrated increased markers of fibrosis and inflammation, especially in the males. Additionally, we found that the beneficial effects of estrogens on adipose tissue require adipocyte ERα. Lastly, we determined the role of ERβ in regulating inflammation and fibrosis, by breeding the AdipoERα into the βERKO background and found that in the absence of adipocyte ERα, ERβ has a protective role. These data suggest that adipose tissue and adipocyte ERα protects against adiposity, inflammation, and fibrosis in both males and females.
Keywords: BAT, brown adipose tissue; ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; Estrogen receptor alpha (ERα); Fibrosis; Inflammation; WAT, white adipose tissue; White adipose tissue (WAT).
Figures
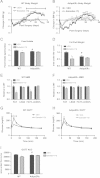
Figure 1
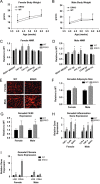
Wild type vs αERKO adipose tissue inflammation and fibrosis. (A and B) Weekly body weight was measured in singly housed female and male mice on normal chow (_n_=8/genotype). (C and D) Body composition was measured by NMR in 6-week-old female and male mice (_n_=8/genotype). (E) Representative photomicrographs of H&E staining of gonadal WAT from 6-week-old male and female mice. (F) Analysis of adipocyte cell size in both male and female of gonadal adipose tissue from 6-week-old mice (_n_=8/genotype). (G) Messenger RNA levels of F4/80 were quantified using qPCR whole adipose tissue from 6-week-old chow-fed females and males (_n_=8/genotype). (H and I) Messenger RNA levels were quantified using qPCR of whole adipose tissue from 6-week-old chow-fed females and males (_n_=8/genotype). Data are presented as mean±SEM, and *P<0.05 between WT and αERKO.
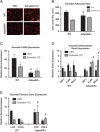
Figure 2
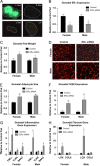
Viral mediated knockdown of ERα in visceral adipose tissue regulates adipocyte size and gene function. Injection methods were determined by using the average surface area and shape of the gonadal adipose tissue. The right gonadal pad that was injected with the control virus is set to 1, and data are normalized to the control-injected pad. (A) Representative images of green fluorescent protein (GFP) in the pad injected with the AAVsiRNA ERα demonstrate that only in the AAVsiRNA ERα GFP-injected pad (L Gon) is GFP visible (the tissue from each view has been outlined for reference), whereas the fat pad injected with the control AAVsiRNA/non-GFP virus is not. Representative photomicrographs of H&E staining of the Gonadal control and siRNA pad from both the males and females at 3 weeks post-injection (_n_=9). (B) Messenger RNA levels of ERα were quantified using qPCR in whole adipose tissue at 3 weeks post-injection of both males and females (_n_=9). (C) Gonadal pad weights of both the control and the ERα siRNA of both males and females were weighed at the time of sacrifice at 3 weeks post-injection (_n_=9). (D) Representative photomicrographs of H&E staining of gonadal WAT from male and female mice. (E) Analysis of adipocyte cell size in both male and female of gonadal adipose tissue (_n_=9). (F) Messenger RNA levels of F4/80 were quantified using qPCR whole adipose tissue at 3 weeks post-injection of both male and female mice (_n_=9). (G and H) Messenger RNA levels of indicated genes were quantified using qPCR in whole adipose tissue at 3 weeks post-injection of both male and female mice (_n_=9). Data are presented as mean±SEM, and *P<0.05 between the ERα siRNA injected pad and the pad injected with the control virus.
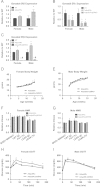
Figure 3
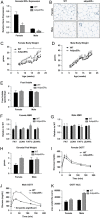
Adipocyte ERα regulates body weight adipocyte distribution. (A) Messenger RNA levels of ERα were quantified using qPCR in collagenase-isolated adipocytes from 18-week-old chow fed females and males (_n_=10/genotype). (B) Immunohistochemistry for ERα from male and female gonadal adipose tissue from WT and AdipoERα mice demonstrating significantly fewer ERα immunoreactive positive adipocytes in the AdipoERα mice relative to WT. Arrows point to ERα positive adipocyte nuclei, whereas the arrowhead demonstrates non-adipocyte nuclei positively stained for ERα demonstrating efficacy of the staining. (C and D) Weekly body weight was measured in singly housed female and male mice on normal chow (_n_=10/genotype). (E) Weekly food intake was measured in singly housed female and male mice on normal chow (_n_=6/genotype). (F and G) Body composition was measured by NMR in 15-week-old male and female mice (_n_=10/genotype). (H) Gonadal fat pad weights in 18 week old females and males (_n_=10/genotype). (I and J) Oral glucose tolerance tests (OGTT) were performed in 15-week-old females and males (_n_=10/genotype). (K) Area under the curve (AUC) from the OGTT's was calculated. Data are presented as mean±SEM, and *P<0.05 between WT and AdipoERα mice.
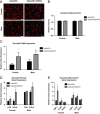
Figure 4
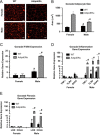
Adipocyte ERα regulates adipocyte size and gene function. (A) Representative photomicrographs of H&E staining of gonadal WAT from 18-week-old male and female mice. (B) Analysis of adipocyte cell size in both male and female of gonadal adipose tissue from 18-week-old mice (_n_=10/genotype). (C) Messenger RNA levels of F4/80 were quantified using qPCR whole adipose tissue from 18-week-old chow-fed females and males (_n_=10/genotype). (D) Messenger RNA levels of inflammatory genes were quantified using qPCR in collagenase-isolated adipocytes from 18-week-old chow-fed females and males (_n_=10/genotype). (E) Messenger RNA levels of fibrosis genes were quantified using qPCR in collagenase-isolated adipocytes from 18-week-old chow-fed females and males (n_=10/genotype). Data are presented as mean±SEM, *P<0.05 between WT and AdipoERα mice, and ♯_P<0.05 between male and female mice of like genotype.
Figure 5
Adipocyte ERα regulates the capacity of estradiol-17β to modulate body weight and adipose tissue distribution. All females were OVX and administered VEH or estradiol-17β at 12 weeks and all post-sacrifice measurements were taken in 18-week-old mice, 42 days post-OVX (_n_=8/genotype). (A and B) Daily body weight was measured in singly housed female mice post-OVX on normal chow (_n_=8/genotype). (C) Daily food intake was measured in singly housed female mice post-OVX on normal chow (_n_=8/genotype). (D and E) Body composition was in 30-day post-OVX chow fed females (_n_=8/genotype). (F) At the time of sacrifice (42 days post-OVX), bilateral gonadal fat pads were weighed. Data presented as a fold change over VEH of pad weight/mouse body weight. (G and H) Oral glucose tolerance test (OGTT) was performed in 16-week-old females (_n_=8/genotype), 30 days post-OVX. (I) Area under the curve (AUC) was calculated for the OGTTs. Data are presented as mean±SEM, and *P<0.05 between WT and AdipoERα mice and # P<0.05 between VEH and 17β-estradiol.
Figure 6
Adipocyte ERα regulates the capacity of estradiol-17β to modulate adipose tissue function, adipocyte size and glucose homeostasis. All females were OVX and administered VEH or estradiol-17β at 12 weeks and all post-sacrifice measurements were taken in 18-week-old mice, 42 days post-OVX (_n_=8/genotype). (A) Representative photomicrographs of H&E staining of gonadal WAT. (B) Analysis of adipocyte cell size in gonadal WAT from 18-week-old mice, 42 days post-OVX (_n_=8/genotype). (C) Messenger RNA levels of F4/80 were quantified using qPCR in whole adipose tissue from 18-week-old chow-fed females (_n_=8/genotype). (D and E) Messenger RNA levels of indicated genes were quantified using qPCR in Collagenase-isolated from 18-week-old chow fed females (n_=8/genotype). Data are presented as mean±SEM, and *P<0.05 between WT and AdipoERα mice and ♯_P<0.05 between VEH and 17β-estradiol.
Figure 7
Contribution of ERβ to the AdipoERα phenotype. (A) ERβ is upregulated in gonadal adipose tissue in male and female AdipoERα mice (_n_=9/genotype, 18-week old at sacrifice). (B) ERα mRNA from isolated adipocytes is reduced to similar levels in AdipoERα and AdipoERα/βERKO mice (_n_=9/genotype). (C) ERβ mRNA from isolated adipocytes is reduced to similar levels in AdipoERα and AdipoERα/βERKO mice (_n_=9–10/genotype). (D and E) Weekly body weight was measured in AdipoERα and AdipoERα/βERKO singly housed female and male mice on normal chow (_n_=6/genotype). (F and G) Body composition was measured by NMR in 15-week-old AdipoERα and AdipoERα/βERKO male and female mice (_n_=9–10/genotype). (H and I) Oral glucose tolerance tests (OGTT) were performed in 15-week-old AdipoERα and AdipoERα/βERKO females and males (_n_=9–10/genotype).
Figure 8
(A) Representative photomicrographs of H&E staining of VISC WAT from 18-week-old male and female mice. (B) Analysis of adipocyte cell size in both AdipoERα and AdipoERα/βERKO male and female of gonadal adipose tissue from 18-week-old mice (_n_=9–10/genotype). (C) Messenger RNA levels of F4/80 were quantified using qPCR whole adipose tissue from 18-week-old AdipoERα and AdipoERα/βERKO chow-fed females and males (_n_=9–10/genotype). (D and E) Messenger RNA levels were quantified using qPCR of whole adipose tissue from 18-week-old AdipoERα and AdipoERα/βERKO chow-fed females and males (_n_=9–10/genotype). Data are presented as mean±SEM, and *P<0.05 between AdipoERα and AdipoERα/βERKO.
Figure 9
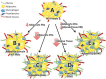
Cartoon depiction of our results. (A) ‘Healthy’ female adipose tissue represented by small (expandable) adipocytes (that contain ERα), low levels of inflammation and fibrosis. (B) Reductions in adipocyte ERα as seen in males (relative to females) and AdipoERα females result in larger (expandable) adipocytes with low levels of inflammation and fibrosis. (C) Knockout of ERα (as seen in AdipoERα males) results in enlarged adipocytes that lack the ability to expand further due to increased levels of inflammation and fibrosis. (D) Knockout of ERα from total adipose tissues results in enlarged adipocytes that lack the ability to expand further due to increased levels of inflammation and fibrosis. (E) Reductions in adipocyte ERα and knockout of adipose tissue ERβ result in adipocytes that lack the ability to expand due to increased levels of inflammation and fibrosis.
Similar articles
- Increased adipose tissue in male and female estrogen receptor-alpha knockout mice.
Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Heine PA, et al. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12729-34. doi: 10.1073/pnas.97.23.12729. Proc Natl Acad Sci U S A. 2000. PMID: 11070086 Free PMC article. - The role of estrogen and estrogen receptor-alpha in male adipose tissue.
Cooke PS, Heine PA, Taylor JA, Lubahn DB. Cooke PS, et al. Mol Cell Endocrinol. 2001 Jun 10;178(1-2):147-54. doi: 10.1016/s0303-7207(01)00414-2. Mol Cell Endocrinol. 2001. PMID: 11403904 Review. - Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta).
Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, Cooke PS. Naaz A, et al. Horm Metab Res. 2002 Nov-Dec;34(11-12):758-63. doi: 10.1055/s-2002-38259. Horm Metab Res. 2002. PMID: 12660895 - Activation of estrogen receptor alpha induces beiging of adipocytes.
Santos RS, Frank AP, Fátima LA, Palmer BF, Öz OK, Clegg DJ. Santos RS, et al. Mol Metab. 2018 Dec;18:51-59. doi: 10.1016/j.molmet.2018.09.002. Epub 2018 Sep 15. Mol Metab. 2018. PMID: 30270132 Free PMC article. - Hypothalamic Estrogen Signaling and Adipose Tissue Metabolism in Energy Homeostasis.
Torres Irizarry VC, Jiang Y, He Y, Xu P. Torres Irizarry VC, et al. Front Endocrinol (Lausanne). 2022 Jun 9;13:898139. doi: 10.3389/fendo.2022.898139. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35757435 Free PMC article. Review.
Cited by
- Anti-Inflammatory and Antioxidant Effects of Regular Consumption of Cooked Ham Enriched with Dietary Phenolics in Diet-Induced Obese Mice.
Serrano A, González-Sarrías A, Tomás-Barberán FA, Avellaneda A, Gironés-Vilaplana A, Nieto G, Ros-Berruezo G. Serrano A, et al. Antioxidants (Basel). 2020 Jul 21;9(7):639. doi: 10.3390/antiox9070639. Antioxidants (Basel). 2020. PMID: 32708089 Free PMC article. - Progress in the molecular understanding of central regulation of body weight by estrogens.
Saito K, Cao X, He Y, Xu Y. Saito K, et al. Obesity (Silver Spring). 2015 May;23(5):919-26. doi: 10.1002/oby.21099. Epub 2015 Apr 10. Obesity (Silver Spring). 2015. PMID: 25865677 Free PMC article. Review. - Developmentally programmed obesity: Is there a role for anti-inflammatory nutritional strategies?
Kearns ML, Reynolds CM. Kearns ML, et al. Exp Physiol. 2024 May;109(5):633-646. doi: 10.1113/EP091209. Epub 2023 Nov 30. Exp Physiol. 2024. PMID: 38031876 Free PMC article. Review. - Perinatal phthalate and high-fat diet exposure induce sex-specific changes in adipocyte size and DNA methylation.
Moody L, Kougias D, Jung PM, Digan I, Hong A, Gorski A, Chen H, Juraska J, Pan YX. Moody L, et al. J Nutr Biochem. 2019 Mar;65:15-25. doi: 10.1016/j.jnutbio.2018.11.005. Epub 2018 Dec 4. J Nutr Biochem. 2019. PMID: 30599393 Free PMC article. - Insights of the role of estrogen in obesity from two models of ERα deletion.
Saavedra-Peña RDM, Taylor N, Rodeheffer MS. Saavedra-Peña RDM, et al. J Mol Endocrinol. 2022 Apr 5;68(4):179-194. doi: 10.1530/JME-21-0260. J Mol Endocrinol. 2022. PMID: 35244608 Free PMC article.
References
- Despres J.P. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9(5):452–459. - PubMed
- Kannel W.B. Regional obesity and risk of cardiovascular disease; the Framingham Study. Journal of Clinical Epidemiology. 1991;44(2):183–190. - PubMed
- Dua A. Leptin: a significant indicator of total body fat but not of visceral fat and insulin insensitivity in African-American women. Diabetes. 1996;45(11):1635–1637. - PubMed
- Kotani K. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. International Journal of Obesity and Related Metabolic Disorders. 1994;18(4) 207–2. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases