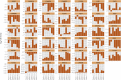Next-generation sequencing of advanced prostate cancer treated with androgen-deprivation therapy - PubMed (original) (raw)
doi: 10.1016/j.eururo.2013.08.011. Epub 2013 Aug 14.
Ian M Sudbery 2, M Eugenia M Villasevil 3, Ernest Mui 4, Janis Fleming 4, Mark Davis 5, Imran Ahmad 3, Joanne Edwards 6, Owen J Sansom 4, David Sims 2, Chris P Ponting 2, Andreas Heger 2, Rhona M McMenemin 7, Ian D Pedley 7, Hing Y Leung 8
Affiliations
- PMID: 24054872
- PMCID: PMC4062940
- DOI: 10.1016/j.eururo.2013.08.011
Next-generation sequencing of advanced prostate cancer treated with androgen-deprivation therapy
Prabhakar Rajan et al. Eur Urol. 2014 Jul.
Abstract
Background: Androgen-deprivation therapy (ADT) is standard treatment for locally advanced or metastatic prostate cancer (PCa). Many patients develop castration resistance (castration-resistant PCa [CRPC]) after approximately 2-3 yr, with a poor prognosis. The molecular mechanisms underlying CRPC progression are unclear.
Objective: To undertake quantitative tumour transcriptome profiling prior to and following ADT to identify functionally important androgen-regulated pathways or genes that may be reactivated in CRPC.
Design, setting, and participants: RNA sequencing (RNA-seq) was performed on tumour-rich, targeted prostatic biopsies from seven patients with locally advanced or metastatic PCa before and approximately 22 wk after ADT initiation. Differentially regulated genes were identified in treatment pairs and further investigated by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) on cell lines and immunohistochemistry on a separate CRPC patient cohort. Functional assays were used to determine the effect of pathway modulation on cell phenotypes.
Outcome measurements and statistical analysis: We searched for gene expression changes affecting key cell signalling pathways that may be targeted as proof of principle in a CRPC in vitro cell line model.
Results and limitations: We identified ADT-regulated signalling pathways, including the Wnt/β-catenin signalling pathway, and observed overexpression of β-catenin in a subset of CRPC by immunohistochemistry. We validated 6 of 12 (50%) pathway members by qRT-PCR on LNCaP/LNCaP-AI cell RNAs, of which 4 (67%) demonstrated expression changes consistent with RNA-seq data. We show that the tankyrase inhibitor XAV939 (which promotes β-catenin degradation) reduced androgen-independent LNCaP-AI cell line growth compared with androgen-responsive LNCaP cells via an accumulation of cell proportions in the G0/G1 phase and reduction in the S and G2/M phases. Our biopsy protocol did not account for tumour heterogeneity, and pathway inhibition was limited to pharmacologic approaches.
Conclusions: RNA-seq of paired PCa samples revealed ADT-regulated signalling pathways. Proof-of-principle inhibition of the Wnt/β-catenin signalling pathway specifically delays androgen-independent PCa cell cycle progression and proliferation and warrants further investigation as a potential target for therapy for CRPC.
Keywords: Androgen-deprivation therapy; Castration resistant; Prostate cancer; Wnt; β-catenin.
Copyright © 2013 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures
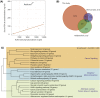
Fig. 1
Differential expression of genes after androgen-deprivation therapy. (A) Correlation between KLK3 messenger RNA transcript expression levels (_x_-axis) normalised by trimmed means of M value in normalised counts per million and serum prostate-specific antigen levels (nanograms per millilitre; _y_-axis). (B) Overlap between genes identified as twofold differentially expressed and previously reported microarray experiments , . (C) Hierarchical clustering of Kyoto Encyclopaedia of Genes and Genomics (KEGG) pathways enriched in upregulated gene sets. Dendrograms show clustering of KEGG terms based on the number of differentially expressed genes two pathways have in common as a proportion of the total number of differentially regulated genes in the two pathways combined. This shows, for example, that the set of cardiac-related pathways cluster with pathways connected to adhesion and matrix interaction. These categories are driven by differential expression of the integrin genes, which are known to be involved in cell–cell and cell–matrix adhesion . FDR = false discovery rate; PSA = prostate-specific antigen.
Fig. 2
Differential expression of genes encoding components of the Wnt/β-catenin–signalling pathway in individual patients. Fold change (logarithmic base 2) after androgen-deprivation therapy of genes in both up- and downregulated gene sets, annotated as being part of the Kyoto Encyclopaedia of Genes and Genomes term Wnt signalling pathway.
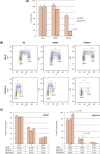
Fig. 3
Inhibition of the Wnt/β-catenin signalling pathway limits growth and delays cell cycle progression of LNCaP-AI cells. (A) WST-1 proliferation assays were performed using either LNCaP cells grown in full medium or LNCaP-AI cells grown in DCC. Cells were treated with 10 μm XAV939 in 0.1% dimethyl sulfoxide (DMSO) or vehicle. Values were normalised to the growth of LNCaP cells in full medium without any treatment. Data from at least three independent experiments were used to obtain the mean relative growth plus or minus standard deviation (SD) for each cell type and treatment condition. (B) Cell cycle analysis was performed using either LNCaP cells grown in full medium or LNCaP-AI cells grown in DCC. Cells were treated with 10 μm XAV939 in 0.1% DMSO or vehicle. Plots shown are representative of at least three independent experiments, from which (C) percentages of cells in each phase of the cell cycle were calculated for each cell type and treatment condition to obtain mean percentage plus or minus SD. BrdU = BrdU fluorescence intensity.
Comment in
- Molecular characterization of prostate cancer following androgen deprivation: the devil in the details.
Barbieri CE, Rubin MA. Barbieri CE, et al. Eur Urol. 2014 Jul;66(1):40-1. doi: 10.1016/j.eururo.2013.08.056. Epub 2013 Aug 30. Eur Urol. 2014. PMID: 24011425 No abstract available.
Similar articles
- Pre-existing Castration-resistant Prostate Cancer-like Cells in Primary Prostate Cancer Promote Resistance to Hormonal Therapy.
Cheng Q, Butler W, Zhou Y, Zhang H, Tang L, Perkinson K, Chen X, Jiang XS, McCall SJ, Inman BA, Huang J. Cheng Q, et al. Eur Urol. 2022 May;81(5):446-455. doi: 10.1016/j.eururo.2021.12.039. Epub 2022 Jan 17. Eur Urol. 2022. PMID: 35058087 Free PMC article. - EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N; European Association of Urology. Heidenreich A, et al. Eur Urol. 2014 Feb;65(2):467-79. doi: 10.1016/j.eururo.2013.11.002. Epub 2013 Nov 12. Eur Urol. 2014. PMID: 24321502 Review. - Transcript Levels of Androgen Receptor Variant 7 and Ubiquitin-Conjugating Enzyme 2C in Hormone Sensitive Prostate Cancer and Castration-Resistant Prostate Cancer.
Lee CH, Ku JY, Ha JM, Bae SS, Lee JZ, Kim CS, Ha HK. Lee CH, et al. Prostate. 2017 Jan;77(1):60-71. doi: 10.1002/pros.23248. Epub 2016 Aug 22. Prostate. 2017. PMID: 27550197 - Upregulated KDM4B promotes prostate cancer cell proliferation by activating autophagy.
Sha J, Han Q, Chi C, Zhu Y, Pan J, Dong B, Huang Y, Xia W, Xue W. Sha J, et al. J Cell Physiol. 2020 Mar;235(3):2129-2138. doi: 10.1002/jcp.29117. Epub 2019 Aug 29. J Cell Physiol. 2020. PMID: 31468537 - A Tale of Two Signals: AR and WNT in Development and Tumorigenesis of Prostate and Mammary Gland.
Pakula H, Xiang D, Li Z. Pakula H, et al. Cancers (Basel). 2017 Jan 27;9(2):14. doi: 10.3390/cancers9020014. Cancers (Basel). 2017. PMID: 28134791 Free PMC article. Review.
Cited by
- Cancer cell plasticity: from cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance.
Bhat GR, Sethi I, Sadida HQ, Rah B, Mir R, Algehainy N, Albalawi IA, Masoodi T, Subbaraj GK, Jamal F, Singh M, Kumar R, Macha MA, Uddin S, Akil ASA, Haris M, Bhat AA. Bhat GR, et al. Cancer Metastasis Rev. 2024 Mar;43(1):197-228. doi: 10.1007/s10555-024-10172-z. Epub 2024 Feb 8. Cancer Metastasis Rev. 2024. PMID: 38329598 Free PMC article. Review. - The lncRNA DRAIC/PCAT29 Locus Constitutes a Tumor-Suppressive Nexus.
Sakurai K, Reon BJ, Anaya J, Dutta A. Sakurai K, et al. Mol Cancer Res. 2015 May;13(5):828-38. doi: 10.1158/1541-7786.MCR-15-0016-T. Epub 2015 Feb 20. Mol Cancer Res. 2015. PMID: 25700553 Free PMC article. - Predicting prostate cancer progression with a Multi-lncRNA expression-based risk score and nomogram integrating ISUP grading.
Ledesma-Bazan S, Cascardo F, Bizzotto J, Olszevicki S, Vazquez E, Gueron G, Cotignola J. Ledesma-Bazan S, et al. Noncoding RNA Res. 2024 Jan 23;9(2):612-623. doi: 10.1016/j.ncrna.2024.01.014. eCollection 2024 Jun. Noncoding RNA Res. 2024. PMID: 38576998 Free PMC article. - Neoadjuvant Treatment of High-Risk, Clinically Localized Prostate Cancer Prior to Radical Prostatectomy.
Pietzak EJ, Eastham JA. Pietzak EJ, et al. Curr Urol Rep. 2016 May;17(5):37. doi: 10.1007/s11934-016-0592-4. Curr Urol Rep. 2016. PMID: 26968417 Review. - Cuprous oxide nanoparticles inhibit prostate cancer by attenuating the stemness of cancer cells via inhibition of the Wnt signaling pathway.
Wang Y, Yang QW, Yang Q, Zhou T, Shi MF, Sun CX, Gao XX, Cheng YQ, Cui XG, Sun YH. Wang Y, et al. Int J Nanomedicine. 2017 Mar 31;12:2569-2579. doi: 10.2147/IJN.S130537. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28408824 Free PMC article.
References
- Center M.M., Jemal A., Lortet-Tieulent J. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. - PubMed
- Scher H.I., Fizazi K., Saad F., AFFIRM Investigators Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. - PubMed
- Tannock I.F., de Wit R., Berry W.R., TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
- Zong Y., Goldstein A.S. Adaptation or selection—mechanisms of castration-resistant prostate cancer. Nat Rev Urol. 2013;10:90–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 15151/CRUK_/Cancer Research UK/United Kingdom
- MC_PC_13077/MRC_/Medical Research Council/United Kingdom
- MC_UU_12021/1/MRC_/Medical Research Council/United Kingdom
- MC_U137761446/MRC_/Medical Research Council/United Kingdom
- 12481/CRUK_/Cancer Research UK/United Kingdom
- MC_EX_UU_G1000902/MRC_/Medical Research Council/United Kingdom
- 15339/CRUK_/Cancer Research UK/United Kingdom
- MC_EX_G1000902/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases