Methylglyoxal induces platelet hyperaggregation and reduces thrombus stability by activating PKC and inhibiting PI3K/Akt pathway - PubMed (original) (raw)
Methylglyoxal induces platelet hyperaggregation and reduces thrombus stability by activating PKC and inhibiting PI3K/Akt pathway
Karin Hadas et al. PLoS One. 2013.
Abstract
Diabetes is characterized by a dysregulation of glucose homeostasis and platelets from patients with diabetes are known to be hyper-reactive and contribute to the accelerated development of vascular diseases. Since many of the deleterious effects of glucose have been attributed to its metabolite methylgyloxal (MG) rather than to hyperglycemia itself, the aim of the present study was to characterize the effects of MG on platelet function. Washed human platelets were pre-incubated for 15 min with MG and platelet aggregation, adhesion on matrix-coated slides and signaling (Western blot) were assessed ex vivo. In vivo, the effect of MG on thrombus formation was determined using the FeCl3-induced carotid artery injury model. MG potentiated thrombin-induced platelet aggregation and dense granule release, but inhibited platelet spreading on fibronectin and collagen. In vivo, MG accelerated thrombus formation but decreased thrombus stability. At the molecular level, MG increased intracellular Ca(2+) and activated classical PKCs at the same time as inhibiting PI3K/Akt and the β3-integrin outside-in signaling. In conclusion, these findings indicate that the enhanced MG concentration measured in diabetic patients can directly contribute to the platelet dysfunction associated with diabetes characterized by hyperaggregability and reduced thrombus stability.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
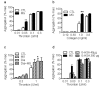
Figure 1. Effect of MG on platelet aggregation.
Aggregation of washed human platelets treated with either solvent (CTL) or methylglyoxal (MG, 1 mmol/L, 15 minutes) prior to the stimulation with either (a) thrombin or (b) collagen. (c) Thrombin-induced aggregation of platelet rich plasma from healthy (CTL) or diabetic patients (Dia) in the absence or in the presence of solvent or MG. (d) Aggregation of washed human platelets treated with either solvent (CTL), methylglyoxal (MG, 1 mmol/L, 15 minutes) or glycated human serum albumin (G-HAS, 40 and 300 µg, 15 minutes) prior to the stimulation with thrombin. The graphs summarise the data from at least 6 different individuals; *P<0.05, **P<0.01 versus CTL.
Figure 2. Effect of MG on platelet [Ca2+]i and degranulation.
(a) Increase in [Ca2+]i measured in washed human platelets treated with either solvent (CTL) or methylglyoxal (MG, 1 mmol/L, 15 minutes) prior to the stimulation with thrombin. (b) Effect of MG pre-treatment on the thrombin (0.03U/ml)-induced release of ATP and (c) on the TRAP-induced surface expression of P-selectin. The graphs summarise the data from at least 6 different individuals; *P<0.05, **P<0.01 versus CTL.
Figure 3. Effect of MG on PKC activation.
(a) Membrane translocation of PKCα and β in washed human platelets stimulated with either methylglyoxal (MG, 1 mmol/L, 15 minutes) or thrombin (0.03U/ml) alone or in combination. (b) Effect of methylglyoxal (MG, 1 mmol/L, 15 and 30 minutes) on the phosphorylation of MLC20 in the absence or in the presence of the PKC inhibitor Ro-318820 (Ro, 300 nM, 30 minutes). (c) Effect of MG on thrombin-induced phosphorylation of MLC20. (d) Effect of Ro-318220 on the thrombin-induced aggregation of washed human platelets treated or not with MG. The graphs summarise the data from 6-8 different experiments; *P<0.05, **P<0.01 versus CTL and # P<0.05, # # P<0.01 versus agonists.
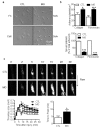
Figure 4. Effect of MG on platelet adhesion, spreading and in vivo thrombus formation.
(a) Representative pictures and (b) quantification of adherent and spread washed human platelets (to fibronectin (Fn)- or collagen (coll)-coated slides) pre-treated with either solvent (CTL) or methylglyoxal (MG, 1 mmol/L, 15 minutes). (c) Representative pictures (upper panel) and quantification (lower graphs) of the effect of in vivo treatment of healthy mice with MG (1 mmol/L, 15 minutes) on thrombus size and time to peak after FeCl3-induced injury of carotid artery. The graphs summarize data obtained in platelets from 12 subjects or 6 animals per group; *P<0.05, ***P<0.001, versus CTL.
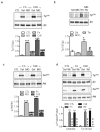
Figure 5. Effect of MG on the phosphorylation of β3 integrin and Akt.
(a) Effect of MG (MG, 1 mmol/L, 15 minutes) on fibronectin (Fn) and collagen (coll)-induced tyrosine phosphorylation of β3 integrin (Tyr747). (b) Effect of MG on thrombin -induced tyrosine phosphorylation (Tyr747) of β3 integrin in washed human platelets. (c) Effect of MG on fibronectin (Fn) and collagen (coll)-induced phosphorylation of Akt (Ser 473). (d) Effect of wortmannin (Wt, 20 nmol/L, 30 minutes) on fibronectin (Fn) and collagen (coll)-induced phosphorylation of β3 integrin (Tyr747) and Akt (Ser 473). The graphs summarise the data from 6 different experiments; *P<0.05, ***P<0.001 versus sol or CTL and # P<0.05, # # # P<0.001 versus agonists.
Similar articles
- Extracellular cyclophilin A activates platelets via EMMPRIN (CD147) and PI3K/Akt signaling, which promotes platelet adhesion and thrombus formation in vitro and in vivo.
Seizer P, Ungern-Sternberg SN, Schönberger T, Borst O, Münzer P, Schmidt EM, Mack AF, Heinzmann D, Chatterjee M, Langer H, Malešević M, Lang F, Gawaz M, Fischer G, May AE. Seizer P, et al. Arterioscler Thromb Vasc Biol. 2015 Mar;35(3):655-63. doi: 10.1161/ATVBAHA.114.305112. Epub 2014 Dec 30. Arterioscler Thromb Vasc Biol. 2015. PMID: 25550208 - Modulation of platelet activation and thrombus formation using a pan-PI3K inhibitor S14161.
Yi W, Li Q, Shen J, Ren L, Liu X, Wang Q, He S, Wu Q, Hu H, Mao X, Zhu L. Yi W, et al. PLoS One. 2014 Aug 12;9(8):e102394. doi: 10.1371/journal.pone.0102394. eCollection 2014. PLoS One. 2014. PMID: 25115838 Free PMC article. - Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice.
Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV. Chen J, et al. Blood. 2004 Sep 15;104(6):1703-10. doi: 10.1182/blood-2003-10-3428. Epub 2004 Apr 22. Blood. 2004. PMID: 15105289 Free PMC article. - Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation.
Harper MT, Poole AW. Harper MT, et al. J Thromb Haemost. 2010 Mar;8(3):454-62. doi: 10.1111/j.1538-7836.2009.03722.x. Epub 2009 Dec 11. J Thromb Haemost. 2010. PMID: 20002545 Review. - Dynamics of platelet thrombus formation.
Jackson SP, Nesbitt WS, Westein E. Jackson SP, et al. J Thromb Haemost. 2009 Jul;7 Suppl 1:17-20. doi: 10.1111/j.1538-7836.2009.03401.x. J Thromb Haemost. 2009. PMID: 19630759 Review.
Cited by
- Increased Dicarbonyl Stress as a Novel Mechanism of Multi-Organ Failure in Critical Illness.
van Bussel BC, van de Poll MC, Schalkwijk CG, Bergmans DC. van Bussel BC, et al. Int J Mol Sci. 2017 Feb 7;18(2):346. doi: 10.3390/ijms18020346. Int J Mol Sci. 2017. PMID: 28178202 Free PMC article. Review. - Shotgun proteomics data on the impact of hyperglycaemia on platelet protein acetylation by aspirin.
Finamore F, Reny JL, Malacarne S, Fontana P, Sanchez JC. Finamore F, et al. Data Brief. 2018 Nov 23;21:2475-2481. doi: 10.1016/j.dib.2018.11.082. eCollection 2018 Dec. Data Brief. 2018. PMID: 30560156 Free PMC article. - CXCL12 regulates platelet activation via the regulator of G-protein signaling 16.
Karim ZA, Alshbool FZ, Vemana HP, Conlon C, Druey KM, Khasawneh FT. Karim ZA, et al. Biochim Biophys Acta. 2016 Feb;1863(2):314-21. doi: 10.1016/j.bbamcr.2015.11.028. Epub 2015 Nov 25. Biochim Biophys Acta. 2016. PMID: 26628381 Free PMC article. - N-acetylcysteine attenuates systemic platelet activation and cerebral vessel thrombosis in diabetes.
Wang B, Yee Aw T, Stokes KY. Wang B, et al. Redox Biol. 2018 Apr;14:218-228. doi: 10.1016/j.redox.2017.09.005. Epub 2017 Sep 20. Redox Biol. 2018. PMID: 28961512 Free PMC article. - Identification of the Antithrombotic Mechanism of Leonurine in Adrenalin Hydrochloride-Induced Thrombosis in Zebrafish via Regulating Oxidative Stress and Coagulation Cascade.
Liao L, Zhou M, Wang J, Xue X, Deng Y, Zhao X, Peng C, Li Y. Liao L, et al. Front Pharmacol. 2021 Nov 4;12:742954. doi: 10.3389/fphar.2021.742954. eCollection 2021. Front Pharmacol. 2021. PMID: 34803688 Free PMC article.
References
- Thornalley PJ (2005) Dicarbonyl intermediates in the maillard reaction. Ann N Y Acad Sci 1043: 111-117. doi:10.1196/annals.1333.014. - DOI - PubMed
- Thornalley PJ (1993) The glyoxalase system in health and disease. Mol Aspects Med 14: 287-371. doi:10.1016/0098-2997(93)90002-U. - DOI - PubMed
- Thornalley PJ (2003) Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans 31: 1343-1348. doi:10.1042/BST0311343. - DOI - PubMed
- Rabbani N, Thornalley PJ (2011) Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol 22: 309-317. doi:10.1016/j.semcdb.2011.02.015. - DOI - PubMed
- Lapolla A, Flamini R, Dalla VA, Senesi A, Reitano R et al. (2003) Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clin Chem Lab Med 41: 1166-1173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study was supported by the Deutsche Forschungsgemeinschaft (SFB 815/A16 and the Exzellenzcluster 147 “Cardio-Pulmonary System”). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous