A DNA break inducer activates the anticodon nuclease RloC and the adaptive immunity in Acinetobacter baylyi ADP1 - PubMed (original) (raw)
A DNA break inducer activates the anticodon nuclease RloC and the adaptive immunity in Acinetobacter baylyi ADP1
Daniel Klaiman et al. Nucleic Acids Res. 2014 Jan.
Abstract
Double-stranded DNA breaks (DSB) cause bacteria to augment expression of DNA repair and various stress response proteins. A puzzling exception educes the anticodon nuclease (ACNase) RloC, which resembles the DSB responder Rad50 and the antiviral, translation-disabling ACNase PrrC. While PrrC's ACNase is regulated by a DNA restriction-modification (R-M) protein and a phage anti-DNA restriction peptide, RloC has an internal ACNase switch comprising a putative DSB sensor and coupled ATPase. Further exploration of RloC's controls revealed, first, that its ACNase is stabilized by the activating DNA and hydrolysed nucleotide. Second, DSB inducers activated RloC's ACNase in heterologous contexts as well as in a natural host, even when R-M deficient. Third, the DSB-induced activation of the indigenous RloC led to partial and temporary disruption of tRNA(Glu) and tRNA(Gln). Lastly, accumulation of CRISPR-derived RNA that occurred in parallel raises the possibility that the adaptive immunity and RloC provide the genotoxicated host with complementary protection from impending infections.
Figures
Figure 1.
_Gka_RloC's ACNase activity is sustained by the activating DNA. (A) The 5′-32-P labelled ACNase substrate 7-2'-Om-Glu-ASL. (B) _Gka_RloC's ACNase was activated for 20 min in the presence of ATP and DNA followed by further pre-incubation with DNase buffer (lanes 1–5), or DNase I buffer containing purified oligonucleotides formed by a DNase I digestion of an activating DNA dose (lanes 6–9) or DNase I (lanes 10–13). In lanes 14–17 DNase I was included in the activation mixture. ACNase activity was subsequently assayed as detailed in ‘Materials and Methods’ section. DDR, DNase-I digest residue; After or During, DNase I added after the activation or during the activation, respectively; ASL, [5′-32P]7-2'-Om-Glu-ASL; 8mer, labelled cleavage product.
Figure 2.
ADP stabilizes _Gka_RloC's activated ACNase. (A) ACNase activation mixtures were supplemented with the indicated amount of ADP and the ACNase assayed using 7-2'-Om-Glu-ASL as a substrate. (B) ADP was added at the indicated amounts after the activation and the ACNase assayed as in A. (C) Initial ACNase reaction rate versus ADP level in the activation mixture (During) or added after the activation (After). (D) ACNase was assayed at the indicated _Gka_RloC levels followed by activation without or with 0.2 mM ADP.
Figure 3.
MMC activates _Gka_RloC's ACNase in E. coli. Escherichia coli Rosetta cells transformed with expression plasmids encoding the indicated _Gka_RloC alleles were incubated in the absence or presence of 200 ng/ml MMC and/or 0.1 mM IPTG. RNA was extracted, separated by denaturing gel electrophoresis and stained with ethidium bromide. _Gka_RloC protein was monitored by western blot using an anti-His6 tag antibody. Full-sized, wt or E696A _Gka_RloC protein; PF, tRNA precursor fragment.
Figure 4.
DSB inducers activate _Aba_RloC in E. coli. (A) Escherichia coli Rosetta cells expressing _Aba_RloC from a plasmid were incubated in the presence or absence of MMC and/or IPTG and in vivo ACNase activity and _Aba_RloC protein monitored as in Figure 3. (B) Similar to (A) except that the cells encoded the indicated _Aba_RloC alleles and were exposed also to 30 µg/ml NAL.
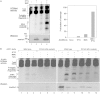
Figure 5.
NAL activates the indigenous _Aba_RloC ACNase. (A) The wild-type or ΔrloC alleles of A. baylyi ADP1 were grown for 4 h at 25°C in the absence or presence of 30 µg/ml of NAL and in vivo ACNase activity monitored as in Figure 3. X indicates the RloC-independent product accumulating in the presence of NAL. (B) The indicated A. baylyi ADP1 alleles were examined for NAL-induced ACNase activity essentially as in panel A. (C) 5′-OH termini in RNA extracted from the indicated A. baylyi ADP1 alleles exposed to NAL were radiolabelled, the RNA separated then on a long gel in which the 3′ fragments generated by RloC were resolved in two major bands designated ‘a’ and ‘b’. The broken lines between panels B and C match the stained fragments with their 5′-end labelled counterparts.
Figure 6.
tRNAGlu and tRNAGln are natural _Aba_RloC substrates. RloC-dependent products ‘a’ and ‘b’ (Figure 5C) labelled at low specific radioactivity (1 Ci/mmol) were subjected to splint ligation (39) using a ligation oligonucleotide probe 5′-labelled at high specific activity (3000 Ci/mmol) and bridging oligonucleotides complementary to 3′ portions of Arg, Gln, Glu or Asp specific A. baylyi ADP1 tRNAs (
Supplementary Table S1
). Following phosphatase treatment the ligation products were separated by denaturing gel electrophoresis and monitored by autoradiography. The splint ligation set-ups shown below identified the tRNAGlu fragment ‘a’ (left) and the tRNAGln fragment ‘b’ (right). The non-specific NAL-induced product X and Xylene cyanoll were used as respective 60 and 30 nt markers.
Figure 7.
Dynamics of RNA species induced by NAL in A. baylyi ADP1. (A) Time course of formation and disappearance of the NAL-induced RNA products. RNA extracted from A. baylyi ADP1 at the indicated times of exposure to 30 µg/ml NAL was 5′-end labelled, separated by denaturing gel electrophoresis and autoradiographed. (B) NAL activates pre-existing _Aba_RloC. The dynamics of RNA cleavage products induced by 60 µg/ml NAL were followed essentially as in A in the absence or presence of 100 µg/ml SH. (C) The NAL-activated indigenous _Aba_RloC does not deplete its tRNA target. RNA extracted from A. baylyi ADP1 at the indicated times of exposure to 30 µg/ml NAL was separated by denaturing gel electrophoresis, stained with EtBr (left panel) and subjected to northern blot analysis using a 5′-labelled DNA probe complementary to tRNAGlu residues 35–72 (right panel). Product X and 3′ tRNA fragments generated by _Aba_RloC were the respective 60 and 42 nt markers.
Figure 8.
X comprises crRNA molecules. (A) CRISPR repeat-spacer unit of A. baylyi ADP1 (top) and the expected crRNA (bottom). The expected site of Cascade-mediated cleavage of the repeat is indicated by the arrow. (B) Splint ligation set-ups. crRNA (gray) and the labelled ligation oligonucleotide probe (underlined) are juxtaposed by a bridging oligonucleotide matching the 3′ proximal repeat portion of crRNA and the ligation partner (Set-up I). The brace sign ( ) indicates that ligation is expected. In the control Set-ups II and III the bridging oligonucleotide contains one or two extra A residues expected to preclude ligation (indicated by X). (C) Ligation products were separated by denaturing gel electrophoresis and monitored by autoradiography. crRNA (X) and xylene cyanoll were respective 60 and 30 nt markers. CRL–crRNA ligated to the probe, SFL–self-folded bridges ligated to the probe.
) indicates that ligation is expected. In the control Set-ups II and III the bridging oligonucleotide contains one or two extra A residues expected to preclude ligation (indicated by X). (C) Ligation products were separated by denaturing gel electrophoresis and monitored by autoradiography. crRNA (X) and xylene cyanoll were respective 60 and 30 nt markers. CRL–crRNA ligated to the probe, SFL–self-folded bridges ligated to the probe.
Similar articles
- The wobble nucleotide-excising anticodon nuclease RloC is governed by the zinc-hook and DNA-dependent ATPase of its Rad50-like region.
Klaiman D, Steinfels-Kohn E, Krutkina E, Davidov E, Kaufmann G. Klaiman D, et al. Nucleic Acids Res. 2012 Sep 1;40(17):8568-78. doi: 10.1093/nar/gks593. Epub 2012 Jun 22. Nucleic Acids Res. 2012. PMID: 22730290 Free PMC article. - RloC: a wobble nucleotide-excising and zinc-responsive bacterial tRNase.
Davidov E, Kaufmann G. Davidov E, et al. Mol Microbiol. 2008 Sep;69(6):1560-74. doi: 10.1111/j.1365-2958.2008.06387.x. Epub 2008 Aug 4. Mol Microbiol. 2008. PMID: 18681940 Free PMC article. - Phage T4-induced DNA breaks activate a tRNA repair-defying anticodon nuclease.
Bitton L, Klaiman D, Kaufmann G. Bitton L, et al. Mol Microbiol. 2015 Sep;97(5):898-910. doi: 10.1111/mmi.13074. Epub 2015 Jun 26. Mol Microbiol. 2015. PMID: 26031711 - Anticodon nucleases.
Kaufmann G. Kaufmann G. Trends Biochem Sci. 2000 Feb;25(2):70-4. doi: 10.1016/s0968-0004(99)01525-x. Trends Biochem Sci. 2000. PMID: 10664586 Review. - Aminoacylation of transfer RNAs with 2-thiouridine derivatives in the wobble position of the anticodon.
Rogers KC, Crescenzo AT, Söll D. Rogers KC, et al. Biochimie. 1995;77(1-2):66-74. doi: 10.1016/0300-9084(96)88106-5. Biochimie. 1995. PMID: 7541255 Review.
Cited by
- Evolutionary Genomics of Defense Systems in Archaea and Bacteria.
Koonin EV, Makarova KS, Wolf YI. Koonin EV, et al. Annu Rev Microbiol. 2017 Sep 8;71:233-261. doi: 10.1146/annurev-micro-090816-093830. Epub 2017 Jun 28. Annu Rev Microbiol. 2017. PMID: 28657885 Free PMC article. Review. - Prophage induction and differential RecA and UmuDAb transcriptome regulation in the DNA damage responses of Acinetobacter baumannii and Acinetobacter baylyi.
Hare JM, Ferrell JC, Witkowski TA, Grice AN. Hare JM, et al. PLoS One. 2014 Apr 7;9(4):e93861. doi: 10.1371/journal.pone.0093861. eCollection 2014. PLoS One. 2014. PMID: 24709747 Free PMC article. - Prokaryotic innate immunity through pattern recognition of conserved viral proteins.
Gao LA, Wilkinson ME, Strecker J, Makarova KS, Macrae RK, Koonin EV, Zhang F. Gao LA, et al. Science. 2022 Aug 12;377(6607):eabm4096. doi: 10.1126/science.abm4096. Epub 2022 Aug 12. Science. 2022. PMID: 35951700 Free PMC article. - Comprehensive classification of ABC ATPases and their functional radiation in nucleoprotein dynamics and biological conflict systems.
Krishnan A, Burroughs AM, Iyer LM, Aravind L. Krishnan A, et al. Nucleic Acids Res. 2020 Oct 9;48(18):10045-10075. doi: 10.1093/nar/gkaa726. Nucleic Acids Res. 2020. PMID: 32894288 Free PMC article. Review. - CRISPR: a new principle of genome engineering linked to conceptual shifts in evolutionary biology.
Koonin EV. Koonin EV. Biol Philos. 2019;34(1):9. doi: 10.1007/s10539-018-9658-7. Epub 2019 Jan 19. Biol Philos. 2019. PMID: 30930513 Free PMC article.
References
- Williams RS, Williams JS, Tainer JA. Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem. Cell Biol. 2007;85:509–520. - PubMed
- Hirano T. At the heart of the chromosome: SMC proteins in action. Nat. Rev. Mol. Cell Biol. 2006;7:311–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous