Maternal depletion of Piwi, a component of the RNAi system, impacts heterochromatin formation in Drosophila - PubMed (original) (raw)
Maternal depletion of Piwi, a component of the RNAi system, impacts heterochromatin formation in Drosophila
Tingting Gu et al. PLoS Genet. 2013.
Abstract
A persistent question in epigenetics is how heterochromatin is targeted for assembly at specific domains, and how that chromatin state is faithfully transmitted. Stable heterochromatin is necessary to silence transposable elements (TEs) and maintain genome integrity. Both the RNAi system and heterochromatin components HP1 (Swi6) and H3K9me2/3 are required for initial establishment of heterochromatin structures in S. pombe. Here we utilize both loss of function alleles and the newly developed Drosophila melanogaster transgenic shRNA lines to deplete proteins of interest at specific development stages to dissect their roles in heterochromatin assembly in early zygotes and in maintenance of the silencing chromatin state during development. Using reporters subject to Position Effect Variegation (PEV), we find that depletion of key proteins in the early embryo can lead to loss of silencing assayed at adult stages. The piRNA component Piwi is required in the early embryo for reporter silencing in non-gonadal somatic cells, but knock-down during larval stages has no impact. This implies that Piwi is involved in targeting HP1a when heterochromatin is established at the late blastoderm stage and possibly also during embryogenesis, but that the silent chromatin state created is transmitted through cell division independent of the piRNA system. In contrast, heterochromatin structural protein HP1a is required for both initial heterochromatin assembly and the following mitotic inheritance. HP1a profiles in piwi mutant animals confirm that Piwi depletion leads to decreased HP1a levels in pericentric heterochromatin, particularly in TEs. The results suggest that the major role of the piRNA system in assembly of heterochromatin in non-gonadal somatic cells occurs in the early embryo during heterochromatin formation, and further demonstrate that failure of heterochromatin formation in the early embryo impacts the phenotype of the adult.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
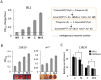
Figure 1. Depletion of functional HP1a in embryos leads to a long-lasting impact on chromatin structure in adult animals.
(A) Quantitative β-galactosidase assays show that reduction of maternally loaded functional HP1a in embryos causes suppression of variegation of the hsp70-lacZ reporter on chromosome 3L (BL1). The crosses used to achieve depletion are shown on the right. Data from adult female flies. (B) Pigment assays and eye pictures show the suppression of variegation effect of maternal depletion of functional HP1a on the hsp70-w reporter 118E10 on chromosome 4, and on wm4 (inversion on chromosome X). Data from adult female flies. (C) HP1a and H3K9me2 enrichment levels in the promoter region of the 118E10 reporter assayed by ChIP-qPCR. Data from mixed adult flies (both males and females). The HP1a enrichment level is normalized to the α-actinin locus. Error bars denote standard error of the mean (SEM). Note that the CyO balancer does not impact variegation of the BL1, 118E10 or wm4 reporters in this genetic background (Figure S1). WT = wild type; C = control; M = maternal depletion; Z = zygotic depletion; M+Z = maternal and zygotic depletion.
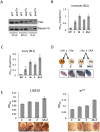
Figure 2. The suppression of variegation in response to Piwi depletion reflects the reduced level of Piwi protein in the early embryo.
(A) Western blot analysis of Piwi proteins shows that piwi2/+ heterozygous female fly ovaries exhibit half the amount of Piwi protein found in wild type (WT). Myosin VI is used as the loading control; the volume of lysate loaded is indicated beneath. (B, C) Quantitative β-galactosidase assays show that decreased maternal loading of Piwi leads to suppression of variegation at the BL1 reporter in both non-gonadal (B) and gonadal (C) cells in adults. (D) X-gal staining in ovaries shows that both maternal and zygotic depletion of Piwi leads to elevated expression of β-galactosidase in ovaries. Depletion strategies are shown in (D). (E) Similarly, a small but consistent loss in silencing is observed on maternal or zygotic depletion of Piwi using an eye phenotype for assessment. piwi mutant alleles used: w; piwi2/CyO for B, C, D and the left panel of E; w; piwi1/CyO for the right panel of E. Error bars denote SEM.
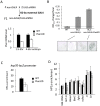
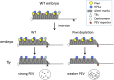
Figure 3. Knockdown (KD) of Piwi in early embryos has a down-stream effect on chromatin structure of a PEV reporter in late stage animals.
(A) Strategies for knocking down the Piwi mRNA in early embryos. By crossing females with the germ line specific GAL4 driver (_nos_-GAL4; NGT) to males with the UAS-shRNA hairpin, Piwi mRNA is depleted in the F1 generation embryo, presumably degraded by the small RNA produced by the paternally-derived shRNA hairpin driven by maternally loaded GAL4. Expression levels are given relative to the RPL32 locus. (B) A quantitative β-galactosidase assay of whole adult males and an X-gal staining assay for β-galactosidase expression in larval imaginal discs both demonstrate that embryonic depletion of Piwi leads to the suppression of variegation of the _hsp70_-lacZ PEV reporter on the Y chromosome (BL2) in subsequent developmental stages. (C) ChIP-quantitative PCR analysis for HP1a enrichment levels at the promoter region of the BL2 reporter in adult males depleted for Piwi in the early embryo. (D) HP1a enrichment levels at other heterochromatic loci in adult males depleted for Piwi in the early embryo. In (C) and (D), ChIP-qPCR was performed using male adult whole flies from mothers having the NGT driver and fathers having the shRNA against Piwi. The enrichment levels are normalized to the α-actinin locus. Error bars denote SEM.
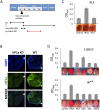
Figure 4. Eye lineage-specific knockdown (KD) of HP1a or EGG, but not of RNAi components Piwi or AGO2, suppresses variegation of a white PEV reporter.
(A) Schematic illustration of the developmental stage of depletion of the protein of interest by null alleles (used in Figures 1 and 2), by the _nos_-GAL4 driver (used in Figure 3), and by the _ey_-GAL4 driver (used here). (B) Immunofluorescent staining of HP1a in the eye disc. HP1a expression is knocked down in the eye lineage using the _ey_-GAL4 driver, and recovered just behind the morphogenetic furrow where _ey_-GAL4 is shut off. Arrows point to the morphogenetic furrow. (C) Pigment assays show that depletion of HP1a by the _ey_-GAL4 driver leads to suppression of variegation of the white gene on the BL2 reporter, while depletion of Piwi does not. Data from adult males. (D) Pigment assay results show that depletion of HP1a or EGG, but not Piwi, in the eye lineage from _ey_-GAL4-driven KD leads to increased expression levels of wm4 and of _hsp70_-w from the 118E10 reporter. Error bars denote SEM. Data from adult females.
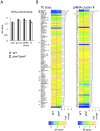
Figure 5. Lower HP1a enrichment at repetitious sequences in piwi2/piwi2 mutant larvae.
(A) HP1a enrichment in heterochromatin regions is slightly lower in piwi2/piwi2 mutant larvae than in wild type. This is most apparent for TEs. WT = wild type; pc.het = pericentric heterochromatin; TE = transposable elements. (B) HP1a enrichment profile for various TE classes and piRNA clusters. The arrows point to the classes of TEs and piRNA clusters mentioned in the text.
Figure 6. Model for Piwi's role in the heterochromatin formation and PEV reporter silencing.
In wild type embryos, Piwi guides HP1a deposition to a subset of TEs in heterochromatin, maintaining normal levels of silent marks. PEV reporter silencing is dependent on the spreading of heterochromatin components from the heterochromatin mass. During development, the silent state is maintained during mitosis independent of Piwi. When Piwi is depleted at the critical stage of heterochromatin establishment in the embryos, the HP1a level in heterochromatin in this region is lower; the reduced HP1a enrichment results in loss of silencing of the sensitive PEV reporter, a state that is propagated to adult animals. Depletion of HP1a in the early embryo has a similar effect, even when HP1a is present at normal levels during mitotic cell divisions. Piwi may act at the border regions between euchromatin and heterochromatin in particular, to prevent aberrant transcription, making PEV reporters particularly sensitive to the depletion of Piwi.
Similar articles
- Drosophila Piwi functions downstream of piRNA production mediating a chromatin-based transposon silencing mechanism in female germ line.
Wang SH, Elgin SC. Wang SH, et al. Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21164-9. doi: 10.1073/pnas.1107892109. Epub 2011 Dec 12. Proc Natl Acad Sci U S A. 2011. PMID: 22160707 Free PMC article. - Piwi Modulates Chromatin Accessibility by Regulating Multiple Factors Including Histone H1 to Repress Transposons.
Iwasaki YW, Murano K, Ishizu H, Shibuya A, Iyoda Y, Siomi MC, Siomi H, Saito K. Iwasaki YW, et al. Mol Cell. 2016 Aug 4;63(3):408-19. doi: 10.1016/j.molcel.2016.06.008. Epub 2016 Jul 14. Mol Cell. 2016. PMID: 27425411 - Piwi Is Required during Drosophila Embryogenesis to License Dual-Strand piRNA Clusters for Transposon Repression in Adult Ovaries.
Akkouche A, Mugat B, Barckmann B, Varela-Chavez C, Li B, Raffel R, Pélisson A, Chambeyron S. Akkouche A, et al. Mol Cell. 2017 May 4;66(3):411-419.e4. doi: 10.1016/j.molcel.2017.03.017. Epub 2017 Apr 27. Mol Cell. 2017. PMID: 28457744 - Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila.
Elgin SC, Reuter G. Elgin SC, et al. Cold Spring Harb Perspect Biol. 2013 Aug 1;5(8):a017780. doi: 10.1101/cshperspect.a017780. Cold Spring Harb Perspect Biol. 2013. PMID: 23906716 Free PMC article. Review. - A novel epigenetic mechanism in Drosophila somatic cells mediated by Piwi and piRNAs.
Lin H, Yin H. Lin H, et al. Cold Spring Harb Symp Quant Biol. 2008;73:273-81. doi: 10.1101/sqb.2008.73.056. Epub 2009 Mar 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19270080 Free PMC article. Review.
Cited by
- Drosophila Heterochromatin Stabilization Requires the Zinc-Finger Protein Small Ovary.
Benner L, Castro EA, Whitworth C, Venken KJT, Yang H, Fang J, Oliver B, Cook KR, Lerit DA. Benner L, et al. Genetics. 2019 Nov;213(3):877-895. doi: 10.1534/genetics.119.302590. Epub 2019 Sep 26. Genetics. 2019. PMID: 31558581 Free PMC article. - Paramutation in Drosophila Requires Both Nuclear and Cytoplasmic Actors of the piRNA Pathway and Induces Cis-spreading of piRNA Production.
Hermant C, Boivin A, Teysset L, Delmarre V, Asif-Laidin A, van den Beek M, Antoniewski C, Ronsseray S. Hermant C, et al. Genetics. 2015 Dec;201(4):1381-96. doi: 10.1534/genetics.115.180307. Epub 2015 Oct 19. Genetics. 2015. PMID: 26482790 Free PMC article. - Maternal Piwi regulates primordial germ cell development to ensure the fertility of female progeny in Drosophila.
Gonzalez LE, Tang X, Lin H. Gonzalez LE, et al. Genetics. 2021 Aug 26;219(1):iyab091. doi: 10.1093/genetics/iyab091. Genetics. 2021. PMID: 34142134 Free PMC article. - siRNAs from an X-linked satellite repeat promote X-chromosome recognition in Drosophila melanogaster.
Menon DU, Coarfa C, Xiao W, Gunaratne PH, Meller VH. Menon DU, et al. Proc Natl Acad Sci U S A. 2014 Nov 18;111(46):16460-5. doi: 10.1073/pnas.1410534111. Epub 2014 Nov 3. Proc Natl Acad Sci U S A. 2014. PMID: 25368194 Free PMC article. - Bioinformatic analyses of sense and antisense expression from terminal inverted repeat transposons in Drosophila somatic cells.
Harrington AW, Steiniger M. Harrington AW, et al. Fly (Austin). 2016 Jan 2;10(1):1-10. doi: 10.1080/19336934.2016.1165372. Epub 2016 Mar 17. Fly (Austin). 2016. PMID: 26986720 Free PMC article.
References
- Heitz E (1928) Das Heterochromatin der Moose. I Jahrb Wiss Botanik 69: 762–818.
- Huisinga KL, Brower-Toland B, Elgin SC (2006) The contradictory definitions of heterochromatin: transcription and silencing. Chromosoma 115: 110–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases