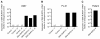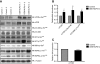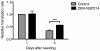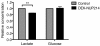Forced expression of the DEK-NUP214 fusion protein promotes proliferation dependent on upregulation of mTOR - PubMed (original) (raw)
Forced expression of the DEK-NUP214 fusion protein promotes proliferation dependent on upregulation of mTOR
Carl Sandén et al. BMC Cancer. 2013.
Abstract
Background: The t(6;9)(p23;q34) chromosomal translocation is found in 1% of acute myeloid leukemia and encodes the fusion protein DEK-NUP214 (formerly DEK-CAN) with largely uncharacterized functions.
Methods: We expressed DEK-NUP214 in the myeloid cell lines U937 and PL-21 and studied the effects on cellular functions.
Results: In this study, we demonstrate that expression of DEK-NUP214 increases cellular proliferation. Western blot analysis revealed elevated levels of one of the key proteins regulating proliferation, the mechanistic target of rapamycin, mTOR. This conferred increased mTORC1 but not mTORC2 activity, as determined by the phosphorylation of their substrates, p70 S6 kinase and Akt. The functional importance of the mTOR upregulation was determined by assaying the downstream cellular processes; protein synthesis and glucose metabolism. A global translation assay revealed a substantial increase in the translation rate and a metabolic assay detected a shift from glycolysis to oxidative phosphorylation, as determined by a reduction in lactate production without a concomitant decrease in glucose consumption. Both these effects are in concordance with increased mTORC1 activity. Treatment with the mTORC1 inhibitor everolimus (RAD001) selectively reversed the DEK-NUP214-induced proliferation, demonstrating that the effect is mTOR-dependent.
Conclusions: Our study shows that the DEK-NUP214 fusion gene increases proliferation by upregulation of mTOR, suggesting that patients with leukemias carrying DEK-NUP214 may benefit from treatment with mTOR inhibitors.
Figures
Figure 1
Expression of the DEK-NUP214 fusion gene. Expression of DEK-NUP214 mRNA by quantitative real-time PCR in the generated stable clones of (A) U937 and (B) PL-21, as well as (C) a patient sample expressing the fusion gene. Values were calculated relative to the expression of the endogenous control, which did not differ between the clones. N.d. denotes not detected.
Figure 2
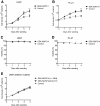
DEK-NUP214 promotes proliferation. Proliferation of (A) U937 and (B) PL-21 cells. Viability of the (C) U937 and (D) PL-21 cell cultures, as determined by trypan blue dye exclusion. (E) Proliferation of U937 cells expressing deletion mutants of DEK-NUP214. Mean values were calculated from three independent experiments, containing all clones. Error bars represent S.E.M.
Figure 3
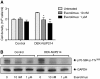
DEK-NUP214 increases the expression of mTOR. A. Phosphorylated and total protein levels for components of the mTOR signaling pathway in U937 cells one day after seeding, showing increased levels of mTOR and activation of the mTORC1 substrate p70S6K without activation of the mTORC2 substrate Akt. α-tubulin is used as an equal loading control. Images show one representative experiment out of three. B. Protein abundance levels of cells expressing DEK-NUP214, relative to control cells. The mean of three experiments, error bars represent S.E.M. C. Expression of the mTOR gene in DEK-NUP214 cells relative to control cells. The mean of three experiments, error bars represent S.E.M.
Figure 4
DEK-NUP214 promotes translation. Global protein synthesis as assayed by the incorporation of radioactively labeled amino acids into newly synthesized protein in U937 cells. All values are normalized to the level of the control cells one day after seeding. Bars represent the mean values of three experiments. Error bars represent S.E.M.
Figure 5
DEK-NUP214 alters the balance between glycolysis and oxidative phosphorylation. Relative concentrations of accumulated lactate and remaining glucose in cell supernatant three days after seeding, showing decreased glycolysis but sustained glucose consumption, indicating increased oxidative phosphorylation in U937 cells expressing DEK-NUP214. Bars represent mean values from three experiments, normalized to control cells. Error bars represent S.E.M.
Figure 6
The proliferative effect of DEK-NUP214 depends on mTOR. A. Proliferation as determined by U937 cell density three days after seeding and with daily additions of the mTORC1 inhibitor everolimus. Viability was unaffected by the treatment. Bars represent mean values from four experiments. Error bars represent S.E.M. B. Potency of the mTORC1 inhibitor everolimus, as determined by reduced phosphorylation of the direct mTORC1 target p70 S6 kinase.
Similar articles
- The DEK oncoprotein and its emerging roles in gene regulation.
Sandén C, Gullberg U. Sandén C, et al. Leukemia. 2015 Aug;29(8):1632-6. doi: 10.1038/leu.2015.72. Epub 2015 Mar 13. Leukemia. 2015. PMID: 25765544 Review. - _DEK-NUP214_-Fusion Identified by RNA-Sequencing of an Acute Myeloid Leukemia with t(9;12)(q34;q15).
Panagopoulos I, Gorunova L, Torkildsen S, Tjønnfjord GE, Micci F, Heim S. Panagopoulos I, et al. Cancer Genomics Proteomics. 2017 Nov-Dec;14(6):437-443. doi: 10.21873/cgp.20053. Cancer Genomics Proteomics. 2017. PMID: 29109093 Free PMC article. Review. - Myelodysplastic syndrome with t(6;9)(p22;q34.1)/DEK-NUP214 better classified as acute myeloid leukemia? A multicenter study of 107 cases.
Fang H, Yabe M, Zhang X, Kim Y, Wu X, Wei P, Chi S, Zheng L, Garcia-Manero G, Shao L, Yuan J, Shen Y, Zheng G, Tang G, Wang W, Loghavi S, Shen Q, Yuan Y, He R, Chen D, Medeiros LJ, Hu S. Fang H, et al. Mod Pathol. 2021 Jun;34(6):1143-1152. doi: 10.1038/s41379-021-00741-w. Epub 2021 Feb 8. Mod Pathol. 2021. PMID: 33558656 - t(6;9)(p22;q34)/DEK-NUP214-rearranged pediatric myeloid leukemia: an international study of 62 patients.
Sandahl JD, Coenen EA, Forestier E, Harbott J, Johansson B, Kerndrup G, Adachi S, Auvrignon A, Beverloo HB, Cayuela JM, Chilton L, Fornerod M, de Haas V, Harrison CJ, Inaba H, Kaspers GJ, Liang DC, Locatelli F, Masetti R, Perot C, Raimondi SC, Reinhardt K, Tomizawa D, von Neuhoff N, Zecca M, Zwaan CM, van den Heuvel-Eibrink MM, Hasle H. Sandahl JD, et al. Haematologica. 2014 May;99(5):865-72. doi: 10.3324/haematol.2013.098517. Epub 2014 Jan 17. Haematologica. 2014. PMID: 24441146 Free PMC article. - Identification of a novel and myeloid specific role of the leukemia-associated fusion protein DEK-NUP214 leading to increased protein synthesis.
Ageberg M, Drott K, Olofsson T, Gullberg U, Lindmark A. Ageberg M, et al. Genes Chromosomes Cancer. 2008 Apr;47(4):276-87. doi: 10.1002/gcc.20531. Genes Chromosomes Cancer. 2008. PMID: 18181180
Cited by
- The DEK oncoprotein and its emerging roles in gene regulation.
Sandén C, Gullberg U. Sandén C, et al. Leukemia. 2015 Aug;29(8):1632-6. doi: 10.1038/leu.2015.72. Epub 2015 Mar 13. Leukemia. 2015. PMID: 25765544 Review. - Leukemia-Associated Nup214 Fusion Proteins Disturb the XPO1-Mediated Nuclear-Cytoplasmic Transport Pathway and Thereby the NF-κB Signaling Pathway.
Saito S, Cigdem S, Okuwaki M, Nagata K. Saito S, et al. Mol Cell Biol. 2016 Jun 15;36(13):1820-35. doi: 10.1128/MCB.00158-16. Print 2016 Jul 1. Mol Cell Biol. 2016. PMID: 27114368 Free PMC article. - Dissecting the Potential Interplay of DEK Functions in Inflammation and Cancer.
Pease NA, Wise-Draper T, Privette Vinnedge L. Pease NA, et al. J Oncol. 2015;2015:106517. doi: 10.1155/2015/106517. Epub 2015 Sep 6. J Oncol. 2015. PMID: 26425120 Free PMC article. Review. - LINC00649 underexpression is an adverse prognostic marker in acute myeloid leukemia.
Guo C, Gao YY, Ju QQ, Zhang CX, Gong M, Li ZL. Guo C, et al. BMC Cancer. 2020 Sep 3;20(1):841. doi: 10.1186/s12885-020-07331-0. BMC Cancer. 2020. PMID: 32883226 Free PMC article. - Imatinib therapy in acute myeloid leukemia with DEK-NUP214 and FIP1L1-PDGFRA rearrangement: A case report.
Yang Y, Lin H, Du Z, Hu R, Tang Y, Liang X, Sun J, Tan Y. Yang Y, et al. Oncol Lett. 2020 May;19(5):3587-3592. doi: 10.3892/ol.2020.11455. Epub 2020 Mar 10. Oncol Lett. 2020. PMID: 32269633 Free PMC article.
References
- Slovak ML, Gundacker H, Bloomfield CD, Dewald G, Appelbaum FR, Larson RA, Tallman MS, Bennett JM, Stirewalt DL, Meshinchi S. et al.A retrospective study of 69 patients with t(6;9)(p23;q34) AML emphasizes the need for a prospective, multicenter initiative for rare ‘poor prognosis’ myeloid malignancies. Leukemia. 2006;20(7):1295–1297. doi: 10.1038/sj.leu.2404233. - DOI - PubMed
- von Lindern M, Fornerod M, van Baal S, Jaegle M, de Wit T, Buijs A, Grosveld G. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol. 1992;12(4):1687–1697. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous