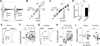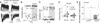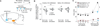Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving - PubMed (original) (raw)
. 2013 Nov;16(11):1644-51.
doi: 10.1038/nn.3533. Epub 2013 Sep 29.
Yao-Ying Ma, Yanhua H Huang, Xiusong Wang, Mami Otaka, Masago Ishikawa, Peter A Neumann, Nicholas M Graziane, Travis E Brown, Anna Suska, Changyong Guo, Mary Kay Lobo, Susan R Sesack, Marina E Wolf, Eric J Nestler, Yavin Shaham, Oliver M Schlüter, Yan Dong
Affiliations
- PMID: 24077564
- PMCID: PMC3815713
- DOI: 10.1038/nn.3533
Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving
Brian R Lee et al. Nat Neurosci. 2013 Nov.
Abstract
In rat models of drug relapse and craving, cue-induced cocaine seeking progressively increases after withdrawal from the drug. This 'incubation of cocaine craving' is partially mediated by time-dependent adaptations at glutamatergic synapses in nucleus accumbens (NAc). However, the circuit-level adaptations mediating this plasticity remain elusive. We studied silent synapses, often regarded as immature synapses that express stable NMDA receptors with AMPA receptors being either absent or labile, in the projection from the basolateral amygdala to the NAc in incubation of cocaine craving. Silent synapses were detected in this projection during early withdrawal from cocaine. As the withdrawal period progressed, these silent synapses became unsilenced, a process that involved synaptic insertion of calcium-permeable AMPA receptors (CP-AMPARs). In vivo optogenetic stimulation-induced downregulation of CP-AMPARs at amygdala-to-NAc synapses, which re-silenced some of the previously silent synapses after prolonged withdrawal, decreased incubation of cocaine craving. Our findings indicate that silent synapse-based reorganization of the amygdala-to-NAc projection is critical for persistent cocaine craving and relapse after withdrawal.
Conflict of interest statement
The authors declare that they do not have any conflicts of interest related to the data presented in this manuscript.
Figures
Figure 1. Recording of BLA-to-NAc excitatory synapses
(A) Diagram showing the position within NAc shell where retrograde tracer FG was stereotaxically injected. (B) Diagram and image showing that NAc shell injection of FG resulted in labeling of BLA neurons. (C) Diagram showing the BLA (circled in yellow), where the ChR2-expressing AAV2 was injected. (D) DIC image showing a brain slice with intra-BLA viral injection. Yellow lines sketch the BLA. (E) Magnified DIC image of a portion of D (red square). (F) YFP image showing fluorescence (viral expression) in the BLA. (G) YPF image showing that intra-BLA injection of ChR2/YFP-expressing virus resulted in expression of ChR2/YFP in neurons and neuronal fibers in the BLA. (H) Example traces showing that optical stimulation elicited action potentials in ChR2-expressing BLA neurons. (I) Example image showing extensive ChR2-expressing fibers in NAc shell in a rat given BLA injection of ChR2/YFP AAV2. (J) Example traces showing elimination of optically-elicited synaptic currents in NAc neurons after perfusion of NBQX. (K) Example EPSCs elicited at BLA-to-NAc synapses by optical stimulations in the minimal stimulation assay showing that failed and successful synaptic responses were readily discernible. (L) Optically evoked individual (gray) and averaged EPSCs (black) from an example NAc neuron showing a short latency of BLA-to-NAc synaptic transmission. Vertical green dashed lines indicate that the latency was measured between the stimulation artifact and initiation of EPSCs. Examples in K and L are taken from saline-experienced control rats (1 day after the end of the training phase).
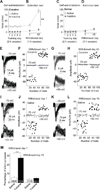
Figure 2. Cocaine self-administration generates silent synapses in the BLA-to-NAc shell projection
(A) Cocaine self-administration training. (B) Incubation of cue-induced cocaine craving: Data are numbers of active and inactive nose-pokes from the same groups of rats in extinction tests performed on withdrawal days 1 and 45 after self-administration training. (C) Saline self-administration training. (D) Extinction tests on withdrawal days 1 and 45 after saline self-administration. (E–H) Levels of silent synapses in BLA-to-NAc projection on withdrawal days 1 and 45 after cocaine self-administration: (E, G) EPSCs (at −70 and +50 mV) evoked by minimal stimulation in representative NAc neurons of rats after 1- or 45-day withdrawal (failures and successes are shown in gray and black traces, respectively). (F, H) Trials of EPSCs from the example neurons in E and G showing a higher failure rate at −70 mV (gray dots represent failures and black dots show successes) on withdrawal day 1 and a reduced failure rate on withdrawal day 45. (I–L) Levels of silent synapses in BLA-to-NAc projection on withdrawal days 1 and 45 after saline self-administration: (I, K) EPSCs (at −70 and +50 mV) evoked by minimal stimulation in representative NAc neurons of rats after 1- or 45-day withdrawal. (J, L) Trials of EPSCs from the example neurons in I and K. (M) Summarized results showing that silent synapses were increased on withdrawal day 1 and returned to the basal level on day 45. Error bar, s.e.m. ** p<0.01, *** p<0.001.
Figure 3. Insertion of CP-AMPARs within BLA-to-NAc synapses after 45 withdrawal days
(A) Example EPSCs elicited at −70 to +50 mV (with 10 mV increment) from BLA-to-NAc synapses; data were collected on withdrawal day 45 in rats previously trained to self-administer saline or cocaine. Whole-cell voltage-clamp recordings were made in the presence of the GABAA receptor-selective antagonist picrotoxin (100 µM) and the NMDA receptor-selective antagonist D-APV (50 µM). (B, C) I–V curves of EPSCs from BLA-to-NAc synapses; the influence of reversal potentials of EPSCs was factored in for each recorded neuron (see Methods). (D) Summary showing that on withdrawal day 45 an increased rectification of EPSCs was detected at BLA-to-NAc synapses from cocaine- but not saline-experienced rats. Scattered dots indicate the values of individual cells. (E–F) Example EPSCs (E) and EPSC peak over trials (F) before and during perfusion of Naspm (a selective antagonist of CP-AMPARs) in saline-trained rats; EPSCs were induced in BLA-to-NAc synapses. (G–H) Example EPSCs (G) and EPSC peak over all trials (H) before and during perfusion of Naspm in cocaine-experienced rats. (I) Summary showing that EPSCs from BLA-to-NAc synapses became sensitive to Naspm on withdrawal day 45 after cocaine self-administration, indicating insertion of CP-AMPARs to BLA-to-NAc excitatory synapses. Error bar, s.e.m. ** p<0.01.
Figure 4. Time courses of cocaine incubation, disappearance of silent synapses, and emergence of CP-AMPARs after withdrawal from cocaine self-administration
(A) Summary showing cue-induced cocaine seeking increased after 10 withdrawal days. Data were from four independent groups; rats given extinction test on withdrawal day 10 but were not tested on day 1. (B) Example EPSCs (left) and their trial course (right) from the minimal stimulation assay of BLA-to-NAc synapses from a rat 10 days after saline self-administration. (C) Example EPSCs (left) and their trial course (right) from the minimal stimulation assay of BLA-to-NAc synapses from a rat 10 days after withdrawal from cocaine self-administration. (D) Summary showing that the level of silent synapses within the BLA-to-NAc projection decreased toward the basal (saline control) level after withdrawal from cocaine. (E–F) Example EPSCs from BLA-to-NAc synapses (left) and their trial course (right) before and during perfusion of Naspm from rats 1 day after withdrawal from saline (E) or cocaine (F) self-administration. (G–H) Example EPSCs from BLA-to-NAc synapses (left) and their trial course (right) before and during perfusion of Naspm from rats 10 days after withdrawal from saline (G) or cocaine (H) self-administration. (I) Summary showing that the sensitivity of EPSCs from BLA-to-NAc synapses to Naspm exhibited a small but significant increase after 10 days of withdrawal from cocaine. (J) Time courses of incubation, disappearance of silent synapses, and insertion of CP-AMPARs after cocaine withdrawal. Data are normalized by setting the withdrawal scores to 0% and withdrawal day 45 scores to 100%. Error bar, s.e.m. * p<0.05, *** p<0.001.
Figure 5. Blockade of CP-AMPARs resilences silent synapses in BLA-to-NAc shell projection on withdrawal day 45
EPSCs elicited by minimal stimulations (recorded at −70 and +50 mV) from BLA-to-NAc synapses 45 days after withdrawal from cocaine self-administration. A–C. Example (A) and trials (B, C) of EPSCs from a cocaine-experienced rat before and during perfusion of Naspm. (D). Summary showing that blockade of CP-AMPARs on withdrawal day 45 caused a re-emergence of silent synapses in the BLA-to-NAc shell projection, suggesting that a large portion of cocaine-generated silent synapses was unsilenced by recruiting CP-AMPARs. Error bar, s.e.m. * p<0.05.
Figure 6. LTD induction at BLA-NAc synapses selectively internalizes CP-AMPARs on withdrawal day 45
(A–B) Example traces (A) and summarized results (B) showing that the LTD induction protocol induced a persistent reduction of the peak amplitudes of EPSCs at BLA-to-NAc shell synapses from cocaine-experienced, but not saline-experienced, rats. Arrows indicate the time points of application of LTD protocols. (C–F) Example EPSCs and the time course showing that BLA-to-NAc shell excitatory synapses, although highly sensitive to Naspm after withdrawal from cocaine (Fig. 3 E–I), became Naspm insensitive after LTD induction (E–G). In saline-experienced rats, EPSCs within this projection were Naspm-insensitive before (Fig. 3 E–I) or after LTD induction (C, D). (G) Summary showing that, at BLA-to-NAc synapses, LTD induction induced a persistent decrease in the peak amplitude of EPSCs, and that EPSCs lost their sensitivity to Naspm after LTD, suggesting a selectively reduction (internalization) of CP-AMPARs from these synapses. Error bar, s.e.m. ** p<0.01.
Figure 7. Reversing the maturation of silent synapses in the BLA-to-NAc projection reverses incubation of cocaine craving
(A) Diagrams showing the timeline of behavioral experiments and the LTD induction protocol in BLA-to-NAc synapses before the test for cue-induced cocaine seeking on withdrawal day 45. (B) Summary showing that after 45 days of withdrawal from cocaine, EPSCs at BLA-to-NAc synapses were significantly inhibited by perfusion of Naspm. After in vivo LTD induction, EPSCs from these synapses became resistant to Naspm. EPSCs were insensitive to Naspm before or after in vivo LTD in saline-experienced rats. (C) In vivo LTD induction prevented incubated cue-induced cocaine seeking in the extinction test. Error bar, s.e.m. ** p<0.01.
Similar articles
- Re-silencing of silent synapses unmasks anti-relapse effects of environmental enrichment.
Ma YY, Wang X, Huang Y, Marie H, Nestler EJ, Schlüter OM, Dong Y. Ma YY, et al. Proc Natl Acad Sci U S A. 2016 May 3;113(18):5089-94. doi: 10.1073/pnas.1524739113. Epub 2016 Apr 18. Proc Natl Acad Sci U S A. 2016. PMID: 27091967 Free PMC article. - Cascades of Homeostatic Dysregulation Promote Incubation of Cocaine Craving.
Wang J, Ishikawa M, Yang Y, Otaka M, Kim JY, Gardner GR, Stefanik MT, Milovanovic M, Huang YH, Hell JW, Wolf ME, Schlüter OM, Dong Y. Wang J, et al. J Neurosci. 2018 May 2;38(18):4316-4328. doi: 10.1523/JNEUROSCI.3291-17.2018. Epub 2018 Apr 6. J Neurosci. 2018. PMID: 29626166 Free PMC article. - AMPA Receptor Plasticity in Accumbens Core Contributes to Incubation of Methamphetamine Craving.
Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, Dolubizno H, Stefanik MT, Murray CH, Sakas C, Wolf ME. Scheyer AF, et al. Biol Psychiatry. 2016 Nov 1;80(9):661-670. doi: 10.1016/j.biopsych.2016.04.003. Epub 2016 Apr 12. Biol Psychiatry. 2016. PMID: 27264310 Free PMC article. - Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond.
Lee BR, Dong Y. Lee BR, et al. Neuropharmacology. 2011 Dec;61(7):1060-9. doi: 10.1016/j.neuropharm.2010.12.033. Epub 2011 Jan 11. Neuropharmacology. 2011. PMID: 21232547 Free PMC article. Review. - Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving.
Loweth JA, Tseng KY, Wolf ME. Loweth JA, et al. Neuropharmacology. 2014 Jan;76 Pt B(0 0):287-300. doi: 10.1016/j.neuropharm.2013.04.061. Epub 2013 May 30. Neuropharmacology. 2014. PMID: 23727437 Free PMC article. Review.
Cited by
- Role of Central Amygdala Neuronal Ensembles in Incubation of Nicotine Craving.
Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Lê AD. Funk D, et al. J Neurosci. 2016 Aug 17;36(33):8612-23. doi: 10.1523/JNEUROSCI.1505-16.2016. J Neurosci. 2016. PMID: 27535909 Free PMC article. - Cocaine Triggers Astrocyte-Mediated Synaptogenesis.
Wang J, Li KL, Shukla A, Beroun A, Ishikawa M, Huang X, Wang Y, Wang YQ, Yang Y, Bastola ND, Huang HH, Kramer LE, Chao T, Huang YH, Sesack SR, Nestler EJ, Schlüter OM, Dong Y. Wang J, et al. Biol Psychiatry. 2021 Feb 15;89(4):386-397. doi: 10.1016/j.biopsych.2020.08.012. Epub 2020 Aug 25. Biol Psychiatry. 2021. PMID: 33069367 Free PMC article. - Eating 'Junk-Food' Produces Rapid and Long-Lasting Increases in NAc CP-AMPA Receptors: Implications for Enhanced Cue-Induced Motivation and Food Addiction.
Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR. Oginsky MF, et al. Neuropsychopharmacology. 2016 Dec;41(13):2977-2986. doi: 10.1038/npp.2016.111. Epub 2016 Jul 7. Neuropsychopharmacology. 2016. PMID: 27383008 Free PMC article. - Cocaine and chronic stress exposure produce an additive increase in neuronal activity in the basolateral amygdala.
Munshi S, Rosenkranz JA, Caccamise A, Wolf ME, Corbett CM, Loweth JA. Munshi S, et al. Addict Biol. 2021 Jan;26(1):e12848. doi: 10.1111/adb.12848. Epub 2019 Nov 21. Addict Biol. 2021. PMID: 31750602 Free PMC article. - FACTORS CONTRIBUTING TO THE ESCALATION OF ALCOHOL CONSUMPTION.
Bowen MT, George O, Muskiewicz DE, Hall FS. Bowen MT, et al. Neurosci Biobehav Rev. 2022 Jan;132:730-756. doi: 10.1016/j.neubiorev.2021.11.017. Epub 2021 Nov 25. Neurosci Biobehav Rev. 2022. PMID: 34839930 Free PMC article. Review.
References
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–456. - PubMed
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. - PubMed
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004;176:101–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA031551/DA/NIDA NIH HHS/United States
- R37 DA023206/DA/NIDA NIH HHS/United States
- DA028020/DA/NIDA NIH HHS/United States
- R01 DA007359/DA/NIDA NIH HHS/United States
- R01 DA009621/DA/NIDA NIH HHS/United States
- DA034856/DA/NIDA NIH HHS/United States
- DA009621/DA/NIDA NIH HHS/United States
- DA014133/DA/NIDA NIH HHS/United States
- DA029565/DA/NIDA NIH HHS/United States
- DA023206/DA/NIDA NIH HHS/United States
- R01 DA014133/DA/NIDA NIH HHS/United States
- R21 DA031551/DA/NIDA NIH HHS/United States
- R01 DA030379/DA/NIDA NIH HHS/United States
- K05 DA029099/DA/NIDA NIH HHS/United States
- DA007359/DA/NIDA NIH HHS/United States
- R01 DA015835/DA/NIDA NIH HHS/United States
- R01 DA023206/DA/NIDA NIH HHS/United States
- P01 DA008227/DA/NIDA NIH HHS/United States
- R37 DA007359/DA/NIDA NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- K99 DA029565/DA/NIDA NIH HHS/United States
- R01 DA034856/DA/NIDA NIH HHS/United States
- F31 DA028020/DA/NIDA NIH HHS/United States
- R37 DA015835/DA/NIDA NIH HHS/United States
- R00 DA029565/DA/NIDA NIH HHS/United States
- DA029099/DA/NIDA NIH HHS/United States
- DA030379/DA/NIDA NIH HHS/United States
- DA036303/DA/NIDA NIH HHS/United States
- R21 DA036303/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous