Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)-THC: mechanism underlying greater toxicity? - PubMed (original) (raw)
Review
Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)-THC: mechanism underlying greater toxicity?
William E Fantegrossi et al. Life Sci. 2014.
Abstract
K2 or Spice products are emerging drugs of abuse that contain synthetic cannabinoids (SCBs). Although assumed by many teens and first time drug users to be a "safe" and "legal" alternative to marijuana, many recent reports indicate that SCBs present in K2 produce toxicity not associated with the primary psychoactive component of marijuana, ∆(9)-tetrahydrocannabinol (Δ(9)-THC). This mini-review will summarize recent evidence that use of K2 products poses greater health risks relative to marijuana, and suggest that distinct pharmacological properties and metabolism of SCBs relative to Δ(9)-THC may contribute to the observed toxicity. Studies reviewed will indicate that in contrast to partial agonist properties of Δ(9)-THC typically observed in vitro, SCBs in K2 products act as full cannabinoid receptor type 1 (CB1R) and type 2 (CB2R) agonists in both cellular assays and animal studies. Furthermore, unlike Δ(9)-THC metabolism, several SCB metabolites retain high affinity for, and exhibit a range of intrinsic activities at, CB1 and CB2Rs. Finally, several reports indicate that although quasi-legal SCBs initially evaded detection and legal consequences, these presumed "advantages" have been limited by new legislation and development of product and human testing capabilities. Collectively, evidence reported in this mini-review suggests that K2 products are neither safe nor legal alternatives to marijuana. Instead, enhanced toxicity of K2 products relative to marijuana, perhaps resulting from the combined actions of a complex mixture of different SCBs present and their active metabolites that retain high affinity for CB1 and CB2Rs, highlights the inherent danger that may accompany use of these substances.
Keywords: CB1 receptors; CB2 receptors; Drug abuse; Drug metabolism; K2/Spice; Synthetic cannabis; ∆(9)-Tetrahydrocannabinol.
© 2013.
Conflict of interest statement
Conflict of Interest Statement:
The authors declare that they have no financial or personal conflicts of interest that influenced, or could be perceived to have influenced, this work.
Figures
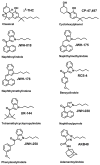
Figure 1. Structures of representative SCBs commonly found in K2 products
Synthetic cannabinoids are derived from diverse structural groups, including classical cannabinoids (Δ9-THC), cyclohexylphenols (CP-47,497), naphthoylindoles (JWH-018), naphthylmethylindoles (JWH-175), naphthylmethylindenes (JWH-176), benzoylindoles (RCS-4), phenylacetylindoles (JWH-250), adamantoylindoles (AKB48), and tetramethylcyclopropylindoles (UR-144).
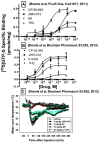
Figure 2. Comparison of the in vivo and in vitro efficacy for SCBs and Δ9-THC
Panel A: CP-55,940, JWH-073 and the M1-metabolite of JWH-073 activate G-proteins with greater efficacy than Δ9-THC. Originally published in PLoS One, 6:e21917, 2011 (Brents et al., 2011). Copyright permission granted by PLoS One open access license. Panel B: CP-55,940, JWH-018 and the M1-metabolite of JWH-018 activate G-proteins more potently and efficaciously than Δ9-THC. Originally published in Biochemical Pharmacology, 83:952, 2012 (Brents et al., 2012). Copyright permission granted by Elsevier. Panel C: JWH-018 and the M1-metabolite of JWH-018 produce greater levels of hypothermia than Δ9-THC. Originally published in Biochemical Pharmacology, 83:952, 2012 (Brents et al., 2012). Copyright permission granted by Elsevier.
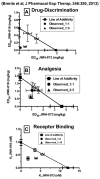
Figure 3. In vivo and in vitro interactions between JWH-018 and JWH-073
Co- administration of JWH-018 and JWH-073 results in synergistic interactions for Δ9-THC drug discrimination (Panel A), analgesia (Panel B) and displacement of radioligand from CB1 receptors (Panel C). Data presented in all panels were originally published in the Journal of Pharmacology and Experimental Therapeutics, 346:350, 2012 (Brents et al., 2013). Copyright permission granted by ASPET.
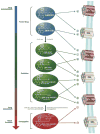
Figure 4. Summary of known metabolism, excretion and downstream cellular signaling activity of JWH-018 and AM2201, two popular SCBs found in K2 products
“+” indicates agonism, “−” indicates antagonism, “X” indicates no binding affinity, “?” indicates not known.
Similar articles
- Pharmacological and Toxicological Effects of Synthetic Cannabinoids and Their Metabolites.
Tai S, Fantegrossi WE. Tai S, et al. Curr Top Behav Neurosci. 2017;32:249-262. doi: 10.1007/7854_2016_60. Curr Top Behav Neurosci. 2017. PMID: 28012093 Free PMC article. Review. - Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications.
Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA. Castaneto MS, et al. Drug Alcohol Depend. 2014 Nov 1;144:12-41. doi: 10.1016/j.drugalcdep.2014.08.005. Epub 2014 Aug 18. Drug Alcohol Depend. 2014. PMID: 25220897 Free PMC article. Review. - Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors.
Rajasekaran M, Brents LK, Franks LN, Moran JH, Prather PL. Rajasekaran M, et al. Toxicol Appl Pharmacol. 2013 Jun 1;269(2):100-8. doi: 10.1016/j.taap.2013.03.012. Epub 2013 Mar 26. Toxicol Appl Pharmacol. 2013. PMID: 23537664 Free PMC article. - Therapeutic potential and safety considerations for the clinical use of synthetic cannabinoids.
Sholler DJ, Huestis MA, Amendolara B, Vandrey R, Cooper ZD. Sholler DJ, et al. Pharmacol Biochem Behav. 2020 Dec;199:173059. doi: 10.1016/j.pbb.2020.173059. Epub 2020 Oct 18. Pharmacol Biochem Behav. 2020. PMID: 33086126 Free PMC article. Review. - Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity.
Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Brents LK, et al. PLoS One. 2011;6(7):e21917. doi: 10.1371/journal.pone.0021917. Epub 2011 Jul 6. PLoS One. 2011. PMID: 21755008 Free PMC article.
Cited by
- NPS: Medical Consequences Associated with Their Intake.
Schifano F, Orsolini L, Papanti D, Corkery J. Schifano F, et al. Curr Top Behav Neurosci. 2017;32:351-380. doi: 10.1007/7854_2016_15. Curr Top Behav Neurosci. 2017. PMID: 27272067 Review. - Cannabinoid and endocannabinoid system: a promising therapeutic intervention for multiple sclerosis.
Khan H, Ghori FK, Ghani U, Javed A, Zahid S. Khan H, et al. Mol Biol Rep. 2022 Jun;49(6):5117-5131. doi: 10.1007/s11033-022-07223-5. Epub 2022 Feb 18. Mol Biol Rep. 2022. PMID: 35182322 Review. - The effects of alcohol and cannabinoid exposure during the brain growth spurt on behavioral development in rats.
Breit KR, Zamudio B, Thomas JD. Breit KR, et al. Birth Defects Res. 2019 Jul 15;111(12):760-774. doi: 10.1002/bdr2.1487. Epub 2019 Mar 10. Birth Defects Res. 2019. PMID: 30854806 Free PMC article. - Synthetic cannabinoid use among patients in residential substance use disorder treatment: prevalence, motives, and correlates.
Bonar EE, Ashrafioun L, Ilgen MA. Bonar EE, et al. Drug Alcohol Depend. 2014 Oct 1;143:268-71. doi: 10.1016/j.drugalcdep.2014.07.009. Epub 2014 Jul 17. Drug Alcohol Depend. 2014. PMID: 25096272 Free PMC article. - Disruption of hippocampal synaptic transmission and long-term potentiation by psychoactive synthetic cannabinoid 'Spice' compounds: comparison with Δ9 -tetrahydrocannabinol.
Hoffman AF, Lycas MD, Kaczmarzyk JR, Spivak CE, Baumann MH, Lupica CR. Hoffman AF, et al. Addict Biol. 2017 Mar;22(2):390-399. doi: 10.1111/adb.12334. Epub 2016 Jan 5. Addict Biol. 2017. PMID: 26732435 Free PMC article.
References
- Aceto MD, Scates SM, Martin BB. Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212-2. Eur J Pharmacol. 2001;416:75–81. - PubMed
- Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–614. - PubMed
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources