Function-structure associations of the brain: evidence from multimodal connectivity and covariance studies - PubMed (original) (raw)
Review
Function-structure associations of the brain: evidence from multimodal connectivity and covariance studies
Jing Sui et al. Neuroimage. 2014.
Abstract
Despite significant advances in multimodal imaging techniques and analysis approaches, unimodal studies are still the predominant way to investigate brain changes or group differences, including structural magnetic resonance imaging (sMRI), functional MRI (fMRI), diffusion tensor imaging (DTI) and electroencephalography (EEG). Multimodal brain studies can be used to understand the complex interplay of anatomical, functional and physiological brain alterations or development, and to better comprehend the biological significance of multiple imaging measures. To examine the function-structure associations of the brain in a more comprehensive and integrated manner, we reviewed a number of multimodal studies that combined two or more functional (fMRI and/or EEG) and structural (sMRI and/or DTI) modalities. In this review paper, we specifically focused on multimodal neuroimaging studies on cognition, aging, disease and behavior. We also compared multiple analysis approaches, including univariate and multivariate methods. The possible strengths and limitations of each method are highlighted, which can guide readers when selecting a method based on a given research question. In particular, we believe that multimodal fusion approaches will shed further light on the neuronal mechanisms underlying the major structural and functional pathophysiological features of both the healthy brain (e.g. development) or the diseased brain (e.g. mental illness) and, in the latter case, may provide a more sensitive measure than unimodal imaging for disease classification, e.g. multimodal biomarkers, which potentially can be used to support clinical diagnosis based on neuroimaging techniques.
Keywords: Brain connectivity; Diffusion MRI; EEG; Multimodal fusion; fMRI; sMRI.
© 2013.
Figures
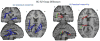
Figure 1. Functional and anatomical connectivity that differs between healthy controls and schizophrenia
(a) Connections between brain regions that was lower in schizophrenia patients than controls (p<0.01) in measure of anatomical connectivity (DTI) (b)Connections between brain regions that differ between SZ and HC in functional connectivity (FMRI). Red lines represent connections for which the functional connectivity was higher in patients, whereas for green line, connectivity was higher in control subjects (p<0.02). IFG, inferior frontal gyrus; IPL, inferior parietal lobule; MTG, middle temporal gyrus; STG, superior temporal gyrus. Ant., anterior;
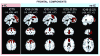
Figure 2. An example of functional-structural connectivity study by (Segall et al., 2012)
The structural (sMRI) components (red) and corresponding rs-fMRI components (blue). The spatial correlation between component pairs is indicated adjacent to the functional component number. Both sMRI and fMRI aggregate components were converted to z-scores and thresholded at Z > 2. Structural components are displayed at the slices with peak activation, indicated as (x, y, z) coordinates in MNI space. Functional components are displayed at different coordinates that best represent their activation.
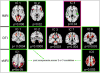
Figure 3. fMRI-sMRI-DTI fusion by mCCA+jICA
Summary of joint and modal specific group discriminative ICs(p<0.05). Joint ICs is significantly group-discriminative in more than 2 modalities, such as IC1, IC2 and IC9. In addition, fMRI_IC4, DTI_IC3 and DTI_IC7 only show significance in a single modality, they are called modal-specific discriminative ICs(pink framed). Hence the modal MCCA+jICA enable people to capture components of interest that are either common or distinct across modalities.
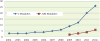
Figure 4. Frequency of published multimodal fusion studies using brain imaging data
Similar articles
- Integrated brain connectivity analysis with fMRI, DTI, and sMRI powered by interpretable graph neural networks.
Qu G, Zhou Z, Calhoun VD, Zhang A, Wang YP. Qu G, et al. Med Image Anal. 2025 Jul;103:103570. doi: 10.1016/j.media.2025.103570. Epub 2025 Apr 9. Med Image Anal. 2025. PMID: 40250104 - Fusing DTI and fMRI data: a survey of methods and applications.
Zhu D, Zhang T, Jiang X, Hu X, Chen H, Yang N, Lv J, Han J, Guo L, Liu T. Zhu D, et al. Neuroimage. 2014 Nov 15;102 Pt 1:184-91. doi: 10.1016/j.neuroimage.2013.09.071. Epub 2013 Oct 5. Neuroimage. 2014. PMID: 24103849 Free PMC article. Review. - Integrated Brain Connectivity Analysis with fMRI, DTI, and sMRI Powered by Interpretable Graph Neural Networks.
Qu G, Zhou Z, Calhoun VD, Zhang A, Wang YP. Qu G, et al. ArXiv [Preprint]. 2024 Aug 26:arXiv:2408.14254v1. ArXiv. 2024. PMID: 39253637 Free PMC article. Updated. Preprint. - Complementary contributions of concurrent EEG and fMRI connectivity for predicting structural connectivity.
Wirsich J, Ridley B, Besson P, Jirsa V, Bénar C, Ranjeva JP, Guye M. Wirsich J, et al. Neuroimage. 2017 Nov 1;161:251-260. doi: 10.1016/j.neuroimage.2017.08.055. Epub 2017 Aug 24. Neuroimage. 2017. PMID: 28842386 - A meta-analysis and systematic review of single vs. multimodal neuroimaging techniques in the classification of psychosis.
Porter A, Fei S, Damme KSF, Nusslock R, Gratton C, Mittal VA. Porter A, et al. Mol Psychiatry. 2023 Aug;28(8):3278-3292. doi: 10.1038/s41380-023-02195-9. Epub 2023 Aug 10. Mol Psychiatry. 2023. PMID: 37563277 Free PMC article.
Cited by
- Combining advanced MRI and EEG techniques better explains long-term motor outcome after very preterm birth.
van 't Westende C, Steggerda SJ, Jansen L, van den Berg-Huysmans AA, van de Pol LA, Wiggers-de Bruine FT, Stam CJ, Peeters-Scholte CMPCD. van 't Westende C, et al. Pediatr Res. 2022 Jun;91(7):1874-1881. doi: 10.1038/s41390-021-01571-x. Epub 2021 May 24. Pediatr Res. 2022. PMID: 34031571 - Dynamic Brain Connectivity in Resting-State FMRI Using Spectral ICA and Graph Approach: Application to Healthy Controls and Multiple Sclerosis.
Riazi AH, Rabbani H, Kafieh R. Riazi AH, et al. Diagnostics (Basel). 2022 Sep 19;12(9):2263. doi: 10.3390/diagnostics12092263. Diagnostics (Basel). 2022. PMID: 36140663 Free PMC article. - Longitudinal brain functional and structural connectivity changes after hemispherotomy in two pediatric patients with drug-resistant epilepsy.
Li Y, Wang Y, Tan Z, Chen Q, Huang W. Li Y, et al. Epilepsy Behav Case Rep. 2018 Nov 24;11:58-66. doi: 10.1016/j.ebcr.2018.11.003. eCollection 2019. Epilepsy Behav Case Rep. 2018. PMID: 30723671 Free PMC article. - Multimodal MRI assessment for first episode psychosis: A major change in the thalamus and an efficient stratification of a subgroup.
Faria AV, Zhao Y, Ye C, Hsu J, Yang K, Cifuentes E, Wang L, Mori S, Miller M, Caffo B, Sawa A. Faria AV, et al. Hum Brain Mapp. 2021 Mar;42(4):1034-1053. doi: 10.1002/hbm.25276. Epub 2020 Dec 30. Hum Brain Mapp. 2021. PMID: 33377594 Free PMC article. - Association between Changes in Cortical Thickness and Functional Connectivity in Male Patients with Alcohol-dependence.
Park SE, Jeon YJ, Baek HM. Park SE, et al. Exp Neurobiol. 2021 Dec 31;30(6):441-450. doi: 10.5607/en21036. Exp Neurobiol. 2021. PMID: 34983884 Free PMC article.
References
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. - PMC - PubMed
- Ardnt C. SPIE Proc. 1996. Information gained by data fusion.
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. - PubMed
- Behrens T, Johansen-Berg H. Diffusion MRI:from quantitative measurement to in-vivo neuroanatomy. Elsevier; 2009.
Publication types
MeSH terms
Grants and funding
- P20 GM103472/GM/NIGMS NIH HHS/United States
- R01 EB005846/EB/NIBIB NIH HHS/United States
- R01EB005846/EB/NIBIB NIH HHS/United States
- R01 EB006841/EB/NIBIB NIH HHS/United States
- R01EB 006841/EB/NIBIB NIH HHS/United States
- R01 EB020407/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical