Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance - PubMed (original) (raw)
. 2013 Oct 15;110(42):17041-6.
doi: 10.1073/pnas.1305170110. Epub 2013 Oct 1.
Shawn F Johnson, Wei Yao, Yu-Chen Li, Young-Eun Choi, Andrea J Bernhardy, Yifan Wang, Marzia Capelletti, Kristopher A Sarosiek, Lisa A Moreau, Dipanjan Chowdhury, Anneka Wickramanayake, Maria I Harrell, Joyce F Liu, Alan D D'Andrea, Alexander Miron, Elizabeth M Swisher, Geoffrey I Shapiro
Affiliations
- PMID: 24085845
- PMCID: PMC3801063
- DOI: 10.1073/pnas.1305170110
Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance
Neil Johnson et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Breast Cancer Type 1 Susceptibility Protein (BRCA1)-deficient cells have compromised DNA repair and are sensitive to poly(ADP-ribose) polymerase (PARP) inhibitors. Despite initial responses, the development of resistance limits clinical efficacy. Mutations in the BRCA C-terminal (BRCT) domain of BRCA1 frequently create protein products unable to fold that are subject to protease-mediated degradation. Here, we show HSP90-mediated stabilization of a BRCT domain mutant BRCA1 protein under PARP inhibitor selection pressure. The stabilized mutant BRCA1 protein interacted with PALB2-BRCA2-RAD51, was essential for RAD51 focus formation, and conferred PARP inhibitor as well as cisplatin resistance. Treatment of resistant cells with the HSP90 inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin reduced mutant BRCA1 protein levels and restored their sensitivity to PARP inhibition. Resistant cells also acquired a TP53BP1 mutation that facilitated DNA end resection in the absence of a BRCA1 protein capable of binding CtIP. Finally, concomitant increased mutant BRCA1 and decreased 53BP1 protein expression occur in clinical samples of BRCA1-mutated recurrent ovarian carcinomas that have developed resistance to platinum. These results provide evidence for a two-event mechanism by which BRCA1-mutant tumors acquire anticancer therapy resistance.
Keywords: cancer therapy; homologous recombination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
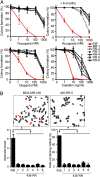
Fig. 1.
MDA-MB-436 clones are resistant to PARP inhibitors and cisplatin. (A) MDA-MB-436 clones RR-1 to RR-6 were significantly more resistant to rucaparib than parental cells (red curve). Cells cultured in the absence of drug for 6 mo remained resistant to rucaparib (+6 mo). Cells were also cross-resistant to olaparib and cisplatin, as measured by colony formation assay (n = 3, mean ± SEM of colonies formed relative to DMSO-treated cells). (B) Metaphase spread analyses of chromosome aberrations and radial formations after treatment with rucaparib (1 µM) for 24 h (n = 3, mean ± SEM). (Inset) Representative metaphase spreads.
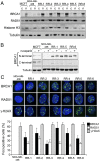
Fig. 2.
Mutant BRCA1 protein is abundant in MDA-MB-436 resistant clones. (A) BRCA1, RAD51, histone H3, and tubulin levels were measured in cytoplasmic (marked as “c”) and nuclear (marked as “n”) extracts from MCF7 cells, MDA-MB-436 parental cells and resistant clones RR-1 to RR-6 by Western blot. (B) MCF7 cells, MDA-MB-436 parental cells and resistant clones RR-1, RR-5, and RR-6 were treated with DMSO (−) or 1 µM rucaparib (+) for 24 h, and BRCA1 protein levels were assessed by using BRCA1 N- or C-terminal–specific antibodies by Western blot. (C) Detection of BRCA1, RAD51, γ-H2AX, and DAPI by immunofluorescence in MDA-MB-436 parental and resistant cells (n = 3, mean ± SEM percentage of cells containing more than five foci). (Inset) Representative cells.
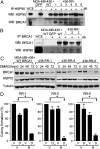
Fig. 3.
HSP90 stabilizes mutant BRCA1. (A) HSP90 was immunoprecipitated from MDA-MB-436 control (GFP) cells, MDA-MB-436+WT cells, and RR clones 1 to 6, and HSP90 and BRCA1 protein levels were analyzed by Western blot (WCE, whole cell extract). (B) BRCA1 was immunoprecipitated from MDA-MB-436 control (GFP) cells, MDA-MB-436+WT cells, and RR clones 1 to 3, and BRCA1 and HSP90 protein levels were analyzed by Western blot. (C) MDA-MB-436+WT, RR-1, RR-5, and RR-6 were treated with 100 nM 17-DMAG for the indicated times, and BRCA1, HSP70, and tubulin protein levels were measured by Western blot. (D) RR-1, RR-5, and RR-6 were treated with vehicle (marked as “V”) or 50 nM 17-DMAG (marked as “D”) in the presence of vehicle (marked as “V”) or 100 nM rucaparib (marked as “R”), and colony formation was assessed (n = 3, mean ± SEM of colonies formed relative to vehicle + vehicle-treated cells).
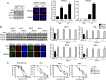
Fig. 4.
Mutant BRCA1 protein promotes RAD51 focus formation. (A) MDA-MB-436 control (GFP) and MDA-MB-436+WT BRCA1 cells (WT) were treated with scrambled (Sc) or 53BP1 siRNA and fixed 6 h after γ-irradiation. RPA32 and RAD51 foci were detected by immunofluorescence. (Left) Western blot demonstrating 53BP1 knockdown and images of representative DAPI-stained cells. (Right) Quantification of RPA32 and RAD51 foci (n = 3, mean ± SEM percentage of cells containing more than five foci). (B) MCF7 and MDA-MB-436 resistant clones RR-1, RR-5, and RR-6 were left untreated (−) or treated with scrambled (Sc) control or three individual BRCA1 siRNAs. After 72 h, cells were fixed 6 h after γ-irradiation treatment. (Left) BRCA1 and RAD51 protein knockdown measured by Western blot and images of representative RR-1 cells after detection of BRCA1, RAD51, γ-H2AX and DAPI by immunofluorescence. (Right) Quantification of foci positive cells (n = 3, mean ± SEM percentage of cells containing more than five foci). (C) MDA-MB-436 parental cells or resistant clones were treated with scrambled (Sc) or three individual BRCA1 siRNAs, exposed to increasing concentrations of rucaparib for 72 h and replated for colony formation. Colony formation was calculated as in Fig. 1_A_ (n = 3, mean ± SEM of colonies formed relative to DMSO-treated cells).
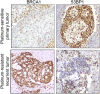
Fig. 5.
Increased mutant BRCA1 protein in a platinum-resistant ovarian carcinoma. BRCA1 and 53BP1 protein levels measured by immunohistochemistry from patient 149101. Representative stains of biopsies taken from the platinum-sensitive primary ovarian tumor and the recurrent resistant tumor.
Similar articles
- Evaluation of candidate biomarkers to predict cancer cell sensitivity or resistance to PARP-1 inhibitor treatment.
Oplustilova L, Wolanin K, Mistrik M, Korinkova G, Simkova D, Bouchal J, Lenobel R, Bartkova J, Lau A, O'Connor MJ, Lukas J, Bartek J. Oplustilova L, et al. Cell Cycle. 2012 Oct 15;11(20):3837-50. doi: 10.4161/cc.22026. Epub 2012 Sep 14. Cell Cycle. 2012. PMID: 22983061 Free PMC article. - Ganetespib overcomes resistance to PARP inhibitors in breast cancer by targeting core proteins in the DNA repair machinery.
Jiang J, Lu Y, Li Z, Li L, Niu D, Xu W, Liu J, Fu L, Zhou Z, Gu Y, Xia F. Jiang J, et al. Invest New Drugs. 2017 Jun;35(3):251-259. doi: 10.1007/s10637-016-0424-x. Epub 2017 Jan 23. Invest New Drugs. 2017. PMID: 28111726 - Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models.
Kim H, Xu H, George E, Hallberg D, Kumar S, Jagannathan V, Medvedev S, Kinose Y, Devins K, Verma P, Ly K, Wang Y, Greenberg RA, Schwartz L, Johnson N, Scharpf RB, Mills GB, Zhang R, Velculescu VE, Brown EJ, Simpkins F. Kim H, et al. Nat Commun. 2020 Jul 24;11(1):3726. doi: 10.1038/s41467-020-17127-2. Nat Commun. 2020. PMID: 32709856 Free PMC article. - BRCA1-Dependent and Independent Recruitment of PALB2-BRCA2-RAD51 in the DNA Damage Response and Cancer.
Foo TK, Xia B. Foo TK, et al. Cancer Res. 2022 Sep 16;82(18):3191-3197. doi: 10.1158/0008-5472.CAN-22-1535. Cancer Res. 2022. PMID: 35819255 Free PMC article. Review. - Chemotherapy for Patients with BRCA1 and BRCA2-Mutated Ovarian Cancer: Same or Different?
Tan DS, Kaye SB. Tan DS, et al. Am Soc Clin Oncol Educ Book. 2015:114-21. doi: 10.14694/EdBook_AM.2015.35.114. Am Soc Clin Oncol Educ Book. 2015. PMID: 25993149 Review.
Cited by
- A Molecular Characterization of the Allelic Expression of the BRCA1 Founder Δ9-12 Pathogenic Variant and Its Potential Clinical Relevance in Hereditary Cancer.
Dominguez-Ortiz J, Álvarez-Gómez RM, Montiel-Manríquez R, Cedro-Tanda A, Alcaraz N, Castro-Hernández C, Bautista-Hinojosa L, Contreras-Espinosa L, Torres-Maldonado L, Fragoso-Ontiveros V, Sánchez-Contreras Y, González-Barrios R, Fuente-Hernández MA, Mejía-Aguayo ML, Juárez-Figueroa U, Padua-Bracho A, Sosa-León R, Obregon-Serrano G, Vidal-Millán S, Núñez-Martínez PM, Pedroza-Torres A, Nicasio-Arzeta S, Rodríguez A, Luna F, Cisneros-Soberanis F, Frías S, Arriaga-Canon C, Herrera-Montalvo LA. Dominguez-Ortiz J, et al. Int J Mol Sci. 2024 Jun 20;25(12):6773. doi: 10.3390/ijms25126773. Int J Mol Sci. 2024. PMID: 38928478 Free PMC article. - Human antigen R and drug resistance in tumors.
Zhou F, Zhang F, Zhou C, Liang M, Cai Z, Lv H, Li W, Wei X. Zhou F, et al. Invest New Drugs. 2019 Oct;37(5):1107-1116. doi: 10.1007/s10637-018-00723-x. Epub 2019 Jan 5. Invest New Drugs. 2019. PMID: 30612309 Review. - State-of-the-art strategies for targeting the DNA damage response in cancer.
Pilié PG, Tang C, Mills GB, Yap TA. Pilié PG, et al. Nat Rev Clin Oncol. 2019 Feb;16(2):81-104. doi: 10.1038/s41571-018-0114-z. Nat Rev Clin Oncol. 2019. PMID: 30356138 Free PMC article. Review. - Targeted blockade of HSP90 impairs DNA-damage response proteins and increases the sensitivity of ovarian carcinoma cells to PARP inhibition.
Gabbasov R, Benrubi ID, O'Brien SW, Krais JJ, Johnson N, Litwin S, Connolly DC. Gabbasov R, et al. Cancer Biol Ther. 2019;20(7):1035-1045. doi: 10.1080/15384047.2019.1595279. Epub 2019 Mar 30. Cancer Biol Ther. 2019. PMID: 30929564 Free PMC article. - Interferon restores replication fork stability and cell viability in BRCA-defective cells via ISG15.
Moro RN, Biswas U, Kharat SS, Duzanic FD, Das P, Stavrou M, Raso MC, Freire R, Chaudhuri AR, Sharan SK, Penengo L. Moro RN, et al. Nat Commun. 2023 Oct 2;14(1):6140. doi: 10.1038/s41467-023-41801-w. Nat Commun. 2023. PMID: 37783689 Free PMC article.
References
- Williams RS, Glover JN. Structural consequences of a cancer-causing BRCA1-BRCT missense mutation. J Biol Chem. 2003;278(4):2630–2635. - PubMed
- Williams RS, et al. Detection of protein folding defects caused by BRCA1-BRCT truncation and missense mutations. J Biol Chem. 2003;278(52):53007–53016. - PubMed
- Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96(22):1659–1668. - PubMed
- Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA83636/CA/NCI NIH HHS/United States
- P50 CA089393/CA/NCI NIH HHS/United States
- P50 CA083636/CA/NCI NIH HHS/United States
- P50 CA090578/CA/NCI NIH HHS/United States
- R01 DK043889/DK/NIDDK NIH HHS/United States
- R01 CA090687/CA/NCI NIH HHS/United States
- R01 CA142698/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous