mTOR regulates phagosome and entotic vacuole fission - PubMed (original) (raw)
mTOR regulates phagosome and entotic vacuole fission
Matej Krajcovic et al. Mol Biol Cell. 2013 Dec.
Abstract
Macroendocytic vacuoles formed by phagocytosis, or the live-cell engulfment program entosis, undergo sequential steps of maturation, leading to the fusion of lysosomes that digest internalized cargo. After cargo digestion, nutrients must be exported to the cytosol, and vacuole membranes must be processed by mechanisms that remain poorly defined. Here we find that phagosomes and entotic vacuoles undergo a late maturation step characterized by fission, which redistributes vacuolar contents into lysosomal networks. Vacuole fission is regulated by the serine/threonine protein kinase mammalian target of rapamycin complex 1 (mTORC1), which localizes to vacuole membranes surrounding engulfed cells. Degrading engulfed cells supply engulfing cells with amino acids that are used in translation, and rescue cell survival and mTORC1 activity in starved macrophages and tumor cells. These data identify a late stage of phagocytosis and entosis that involves processing of large vacuoles by mTOR-regulated membrane fission.
Figures
FIGURE 1:
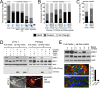
Cell engulfment rescues the effects of amino acid deprivation. (A) Engulfment of apoptotic corpses rescues macrophages from amino acid deprivation–induced cell death. Fates of J774.1 macrophages cultured in full and amino acid (aa)–free media in the presence or absence of apoptotic corpses, determined by time-lapse microscopy for 48 h. Single, single cells, n = 90 for full and 90 for aa-free media; engulfed beads, cells that engulfed from one to five latex beads, n = 60; single neighbors, single cells within same microscopic fields as corpse-engulfing cells, n = 168; engulfed corpse, cells with one or two corpses engulfed before start of time lapse, n = 90; continual engulfment, cells supplied with corpses that were engulfed continuously throughout the time lapse, n = 74. *p < 0.02, **p < 0.001 (when compared with single cells in aa-free media; chi-squared). Data are from at least three independent experiments. (B) Entosis rescues MCF10A cells from the effects of amino acid deprivation. Fates of MCF10A cells (single) and MCF10A cells with an entotic cell corpse (entotic) in aa-free media time lapsed for 48 h (control and FIP200 siRNA [FIP200 si]–treated cells) or 18 h (chloroquine-treated cells). Control single cells, n = 360; control entotic, n = 137; _FIP200_si single, n = 558; _FIP200_si entotic, n = 179; chloroquine single, n = 92; chloroquine entotic, n = 37. *p < 0.002, **p < 0.001 (chi-squared). Data are from at least three independent experiments. (C) Entotic MCF-7 cells (n = 192) harboring an entotic corpse are rescued from cell death and proliferation arrest compared with single control cells (n = 567) in aa-free media. p < 0.001 (chi-squared). Cells were examined for 48 h by time-lapse microscopy. Data are from at least three independent experiments. (D) mTORC1 is reactivated in aa-free media by corpse digestion in J774.1 macrophages (left blots) and primary bone marrow–derived macrophages (right blots). Western blots show restoration of phosphorylated S6-kinase threonine 389 (pS6K) by apoptotic corpse engulfment but not latex bead engulfment in macrophages cultured in aa-free media. pS6K restoration is blocked by treatment with the mTOR inhibitor Torin1 and the lysosome inhibitor ConA. Torin1 was added to cultures 1 h before cell lysis; ConA was added for the duration of the experiment. Untreated macrophages digest the corpse-specific marker H2B-mCherry into free mCherry protein, which is inhibited by ConA treatment. Images show apoptotic corpse expressing H2B-mCherry (red fluorescence, arrow) engulfed by J774.1 macrophage. Bar, 10 μm. (E) mTORC1 is reactivated by entosis. Western blots show higher levels of pS6K in MCF-7 cells cultured in aa-free media under control conditions, with 15% of cells harboring entotic corpses (quantified in graph; representative of two independent experiments), compared with entosis-inhibited conditions with Y-27632 treatment. Images show entotic cell corpses (white arrows) in control cultures, which are absent from Y-27632–treated cultures. Immunofluorescence staining for Lamp1 (red) and β-catenin (green) and 4′,6-diamidino-2-phenylindole (DAPI)–stained nuclei (blue). Bar, 10 μm.
FIGURE 2:
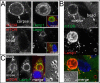
mTOR is recruited to lysosomal vacuoles harboring engulfed cells but not latex beads. (A) Top, mTOR (left) is recruited to corpse-containing entotic vacuole (arrow) (MCF10A), where it colocalizes with Lamp1 (right); inset, colocalization of immunostained mTOR (red) and Lamp1 (green). DAPI-stained nucleus is shown in blue. Bottom, mTOR does not recruit to bead-containing vacuole (left, arrow), which is marked by Lamp1 (right) (MCF10A); inset, merged image with DAPI-stained nucleus in blue. All are confocal microscopic images. Bars, 10 μm. (B) mTOR localizes to corpse-containing entotic vacuole (labeled corpse with arrow; 70% were positive for mTOR, n = 37) but not to bead-containing lysosomal vacuole (bead, arrow; 2.3% positive for mTOR, n = 84) in the same MCF10A cell. Top and middle, confocal images of immunofluorescence for mTOR and Lamp1; bottom, merge with DAPI-stained nucleus (blue); inset, DIC. (C) mTOR localizes to apoptotic cell phagosomes (52% positive for mTOR, n = 54) but not latex bead phagosomes (1.9% positive for mTOR, n = 154) in J774.1 macrophages. Confocal microscopic images show macrophage with an engulfed apoptotic corpse and two beads, as indicated, stained for mTOR and Lamp1 by immunofluorescence. Right, merged image with DAPI-stained nucleus (blue) and DIC.
FIGURE 3:
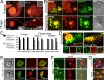
Phagosomes and entotic vacuoles undergo fission. (A) mCherry fluorescence from an entotic corpse expressing H2B-mCherry (top) or an apoptotic corpse expressing H2B-mCherry (bottom) appears as puncta in the cytoplasm of engulfing cells (right) as entotic vacuoles (white dashed circle) and phagosomes (blue circle) shrink over time. Images from time-lapse analysis show mCherry fluorescence (red); insets, DIC; arrows indicate corpses. Note that entotic engulfing cell in top is binucleate and also expresses H2B-mCherry. Bars, 10 μm. See Supplemental Videos S1 and S2. (B) mCherry puncta colocalize with Lamp1-GFP in entotic cells (top) and LysoTracker in primary macrophages (bottom). Maximum projection images of mCherry fluorescence (red), Lamp1-GFP and LysoTracker green (green), and merged and DIC images as indicated; arrows indicate cell corpses. Bars, 10 μm. (C) Quantification of colocalization between mCherry and Lamp1 or LysoTracker in individual phagocytic or entotic engulfing cells. Black bars, percentage of mCherry puncta that colocalize with Lamp1 or LysoTracker; gray bars, percentage of Lamp1 or LysoTracker vesicles that colocalize with mCherry. Percentages for three individual engulfing cells for entosis (MCF10A) or phagocytosis (J774.1 and primary macrophages) are shown. For entosis, cell 1, n = 124 vesicles (black bar) and 137 vesicles (gray bar); cell 2, n = 132, 137; and cell 3, n = 131, 125. For J774.1 phagocytosis, cell 1, n = 66, 83; cell 2, n = 54, 76; and cell 3, n = 55, 67. For phagocytosis with primary macrophages, cell 1, n = 96, 100; cell 2, n = 174, 171; cell 3, n = 157, 143. (D) Corpse-derived mCherry fluorescence redistributes from the entotic vacuole to the lysosome network of the engulfing cell. Maximum projections from confocal time-lapse analysis of a Lamp1-GFP–expressing MCF10A cell with an engulfed entotic corpse expressing H2B-mCherry. Note that Lamp1-GFP–labeled lysosomes acquire red fluorescence over time as entotic vacuole undergoes fission. Top, merged green and red fluorescence of whole cell outlined with hatched white line. Bottom, individual and merged fluorescent channels at time 0 and 10 h for an area of cell cytoplasm. Bar, 10 μm. See Supplemental Video S3. (E) Entotic vacuole, labeled with Lamp1-GFP (green), accumulates 10-kDa red fluorescent dextran (arrowhead) from media over the course of 10 h as the vacuole shrinks in size. Also see Supplemental Figure S5a. (F) An entotic vacuole (arrow) in brefeldin A–treated cells fuses with Lamp1-GFP–labeled lysosomes (green) after polyethylene glycol (PEG)–initiated cell fusion. Arrowhead indicates Lamp1-GFP accumulation at entotic vacuole. Time indicates minutes after cell fusion. Also see Supplemental Figure S5, b and c. (G) Brefeldin A treatment as in F disrupts the Golgi, as shown by GM130 immunostaining (green) in mixed cultures used for cell fusion in F. β-Catenin immunostaining and H2B-mCherry are shown in red. Note that the H2B-mCherry–expressing cells used in this experiment do not express Lamp1-GFP (green), so cells with red nuclei show only GM130 immunofluorescence in the green channel, whereas cells without red nuclei show GM130 immunofluorescence and Lamp1-GFP fluorescence in the green channel.
FIGURE 4:
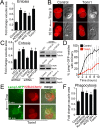
Entotic vacuole and phagosome fission is regulated by mTOR. (A) Inhibitors of mTOR slow entotic vacuole shrinkage. Fold change of vacuole area after 10 h for entotic vacuoles (MCF10A cells) measured by time-lapse microscopy of control and mTOR inhibitor–treated cells cultured in full media. Error bars, SEM for n = 3 independent experiments. Total cell number analyzed for control, n = 57; AZD8055, n = 55; Ku-0063794, n = 55; WYE-354, n = 51; Torin1, n = 55. *p < 0.02 (Student's t test). (B) mTOR inhibition slows vacuole fission. Control entotic vacuole (left) undergoes rapid shrinkage, producing mCherry-labeled puncta as corpse degrades, whereas mTOR-inhibited cell (right) is delayed for fission, leaving a large vacuole as the corpse degrades. Images show mCherry fluorescence (red) and DIC. Dashed line, entotic vacuoles marked by mCherry. Bar, 10 μm. See Supplemental Videos S7 and S8. (C) shRNA- and siRNA-mediated knockdowns of mTOR and Raptor slow entotic vacuole shrinkage. Fold change of vacuole area after 10 h for entotic vacuoles (MCF10A cells) determined by time-lapse microscopy of control and mTOR or Raptor-knockdown cells. Western blots to the right of graph show protein knockdowns. Error bars, SEM for three independent experiments. Total cell number analyzed for pLKO, n = 121; mTOR #1 shRNA, n = 89; mTOR #2 shRNA, n = 131; control (nontargeting siRNA), n = 93; mTOR siRNA, n = 87; Raptor siRNA, n = 97. *p < 0.02 (Student's t test). (D) Torin1 treatment slows the redistribution of corpse-derived mCherry from the entotic vacuole to the lysosome network of engulfing cells. Quantification of percentage of Lamp1-GFP–labeled lysosomes with mCherry fluorescence in four individual control and Torin1-treated cells, examined by confocal microscopy and quantified in every _z_-plane at 0.5-μm intervals through the entire cell every hour for 10 h. Error bars, SD. After 10 h Torin1-treated cells have significantly less mCherry fluorescence in lysosome networks (p < 0.01; Student's t test). (E) An entotic vacuole (arrow) in Torin1-treated cells fuses with Lamp1-GFP–labeled lysosomes (green) after PEG-initiated cell fusion. Arrowhead, Lamp1-GFP accumulation at entotic vacuole. Time indicates minutes after cell fusion. Also see Supplemental Figure S5D and Supplemental Video S9. (F) mTOR inhibition slows phagosome shrinkage in full media. Fold change of phagosome area after 2 h. Error bars, SEM for three independent experiments. Total cell number analyzed for control, n = 50, AZD8055; n = 47, Ku-0063794, n = 30; WYE-354, n = 38; Torin1, n = 54. *p < 0.02 (Student's t test).
FIGURE 5:
Model for phagosome and entotic vacuole fission. The degradation of cell corpses after phagocytosis or entosis recruits mTORC1 (yellow) to Lamp1-positive (green) vacuole membranes. mTORC1 regulates vacuolar fission, which redistributes the luminal contents of phagosomes and entotic vacuoles into the lysosome network of engulfing cells. mTORC1-regulated fission (thick arrow) accelerates vacuole shrinkage by overcoming a constant rate of lysosome fusion (thin arrow). Amino acid export from phagosomes and entotic vacuoles likely provides the survival advantage of engulfing cells in amino acid–free media.
Similar articles
- Amino acids and mechanistic target of rapamycin regulate the fate of live engulfed cells.
Kim SE, Zhang J, Jiang E, Overholtzer M. Kim SE, et al. FASEB J. 2021 Oct;35(10):e21909. doi: 10.1096/fj.202100870R. FASEB J. 2021. PMID: 34547144 Free PMC article. - PIKfyve Regulates Vacuole Maturation and Nutrient Recovery following Engulfment.
Krishna S, Palm W, Lee Y, Yang W, Bandyopadhyay U, Xu H, Florey O, Thompson CB, Overholtzer M. Krishna S, et al. Dev Cell. 2016 Sep 12;38(5):536-47. doi: 10.1016/j.devcel.2016.08.001. Dev Cell. 2016. PMID: 27623384 Free PMC article. - Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes.
Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M. Florey O, et al. Nat Cell Biol. 2011 Oct 16;13(11):1335-43. doi: 10.1038/ncb2363. Nat Cell Biol. 2011. PMID: 22002674 Free PMC article. - Autophagy proteins in macroendocytic engulfment.
Florey O, Overholtzer M. Florey O, et al. Trends Cell Biol. 2012 Jul;22(7):374-80. doi: 10.1016/j.tcb.2012.04.005. Epub 2012 May 19. Trends Cell Biol. 2012. PMID: 22608991 Free PMC article. Review. - How nascent phagosomes mature to become phagolysosomes.
Fairn GD, Grinstein S. Fairn GD, et al. Trends Immunol. 2012 Aug;33(8):397-405. doi: 10.1016/j.it.2012.03.003. Epub 2012 May 3. Trends Immunol. 2012. PMID: 22560866 Review.
Cited by
- Detecting cell-in-cell structures in human tumor samples by E-cadherin/CD68/CD45 triple staining.
Huang H, Chen A, Wang T, Wang M, Ning X, He M, Hu Y, Yuan L, Li S, Wang Q, Liu H, Chen Z, Ren J, Sun Q. Huang H, et al. Oncotarget. 2015 Aug 21;6(24):20278-87. doi: 10.18632/oncotarget.4275. Oncotarget. 2015. PMID: 26109430 Free PMC article. - The Emerging Hallmarks of Cancer Metabolism.
Pavlova NN, Thompson CB. Pavlova NN, et al. Cell Metab. 2016 Jan 12;23(1):27-47. doi: 10.1016/j.cmet.2015.12.006. Cell Metab. 2016. PMID: 26771115 Free PMC article. Review. - Entosis Acts as a Novel Way within Sertoli Cells to Eliminate Spermatozoa in Seminiferous Tubule.
Ahmed N, Yang P, Huang Y, Chen H, Liu T, Wang L, Nabi F, Liu Y, Chen Q. Ahmed N, et al. Front Physiol. 2017 May 30;8:361. doi: 10.3389/fphys.2017.00361. eCollection 2017. Front Physiol. 2017. PMID: 28611685 Free PMC article. - Quantitative proteome analysis of temporally resolved phagosomes following uptake via key phagocytic receptors.
Dill BD, Gierlinski M, Härtlova A, Arandilla AG, Guo M, Clarke RG, Trost M. Dill BD, et al. Mol Cell Proteomics. 2015 May;14(5):1334-49. doi: 10.1074/mcp.M114.044594. Epub 2015 Mar 9. Mol Cell Proteomics. 2015. PMID: 25755298 Free PMC article. - A BORC-dependent molecular pathway for vesiculation of cell corpse phagolysosomes.
Fazeli G, Levin-Konigsberg R, Bassik MC, Stigloher C, Wehman AM. Fazeli G, et al. Curr Biol. 2023 Feb 27;33(4):607-621.e7. doi: 10.1016/j.cub.2022.12.041. Epub 2023 Jan 17. Curr Biol. 2023. PMID: 36652947 Free PMC article.
References
- Botelho RJ, Hackam DJ, Schreiber AD, Grinstein S. Role of COPI in phagosome maturation. J Biol Chem. 2000;275:15717–15727. - PubMed
- Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr Biol. 2005;15:360–365. - PubMed
- Chen D, Xiao H, Zhang K, Wang B, Gao Z, Jian Y, Qi X, Sun J, Miao L, Yang C. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science. 2010;327:1261–1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA148967/CA/NCI NIH HHS/United States
- CA177697/CA/NCI NIH HHS/United States
- R21 CA177697/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U54 CA148967/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous