Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology - PubMed (original) (raw)
Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology
Jiaping Gu et al. J Biol Chem. 2013.
Abstract
Aggregated Tau proteins are hallmarks of Alzheimer disease and other tauopathies. Recent studies from our group and others have demonstrated that both active and passive immunizations reduce Tau pathology and prevent cognitive decline in transgenic mice. To determine the efficacy and safety of targeting the prominent 396/404 region, we developed two novel monoclonal antibodies (mAbs) with distinct binding profiles for phospho and non-phospho epitopes. The two mAbs significantly reduced hyperphosphorylated soluble Tau in long term brain slice cultures without apparent toxicity, suggesting the therapeutic importance of targeting the 396/404 region. In mechanistic studies, we found that neurons were the primary cell type that internalized the mAbs, whereas a small amount of mAbs was taken up by microglia cells. Within neurons, the two mAbs were highly colocalized with distinct pathological Tau markers, indicating their affinity toward different stages or forms of pathological Tau. Moreover, the mAbs were largely co-localized with endosomal/lysosomal markers, and partially co-localized with autophagy pathway markers. Additionally, the Fab fragments of the mAbs were able to enter neurons, but unlike the whole antibodies, the fragments were not specifically localized in pathological neurons. In summary, our Tau mAbs were safe and efficient to clear pathological Tau in a brain slice model. Fc-receptor-mediated endocytosis and the endosome/autophagosome/lysosome system are likely to have a critical role in antibody-mediated clearance of Tau pathology.
Keywords: Alzheimer Disease; Antibodies; Autophagosomes; Endosomes; Immunotherapy; Lysosomes; Phosphorylation; Tau.
Figures
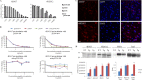
FIGURE 1.
Characterization of newly generated monoclonal Tau antibodies, 4E6G7 and 6B2G12, against the 396/404 Tau region. A, ELISA data of the two mAbs recognizing different Tau epitopes at serial dilutions from 1 mg/ml. 4E6G7 had higher affinity toward the p396/404 and the p404 peptides than the non-Ser(P)-396/404 peptide (non-Ser(P)-396/404 versus Ser(P)-396/404: p < 0.01 from 1/3,000 to 1/243,000; non-Ser(P)-396/404 versus Ser(P)-404: p < 0.01 from 1/3,000 to 1/243,000) and showed little binding to the Ser(P)-396 peptide (Ser(P)-396 versus any other peptide: p < 0.001 at all dilutions; n = 3 in each group). 6B2G12 detected both phospho and non-phospho epitopes to a similar extent with no significant differences between the peptides. B, competitive ELISA of the two mAbs preincubated with the Ser(P)-396/404 or non-Ser(P)-396/404 peptides. The Ser(P)-396/404 peptide blocked the binding of both mAbs to all Tau peptides. The non-Ser(P)-396/404 peptide partially blocked the binding of 6B2G12 to the same peptide and the Ser(P)-396 and the Ser(P)-404 peptides but had limited if any effect on binding of 4E6G7. We did not test 4E6G7 binding to Ser(P)-396 in this experiment because 4E6G7 has very low affinity toward it. C, staining of JNPL3 mouse brain sections by the two mAbs in the cortical region. Scale bar = 20 μm. D, Western blots of the two mAbs detecting low speed supernatant (soluble fraction) of brain homogenate from wild-type (WT) and JNPL3 (transgenic (Tg)) mice. The same samples were probed with PHF1 and Tau5 for comparison. Both 4E6G7 and PHF1, but not 6B2G12 and Tau5, gave significantly greater signal in JNPL3 mice compared with WT mice (*, p < 0.05; **, p < 0.01, n = 5 Tg, n = 3 WT).
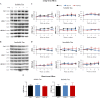
FIGURE 2.
The monoclonal antibodies reduced Tau hyperphosphorylation in long term brain slice culture. A and B, representative images of Western blot analysis of soluble and Sarkosyl-insoluble Tau fractions from brain slices treated with the respective antibodies. The blots were detected by PHF1 recognizing Ser(P)-396/404 epitope, CP27 for total human Tau, and Tau5 for total Tau. C, quantified results showing the change of hyperphosphorylated Tau level and human Tau level. In control slices, the soluble Tau fraction exhibited a ∼60% increase of PHF1/total Tau ratio. Our two monoclonal antibodies, 4E6G7 and 6B2G12, reduced PHF1 immunoreactivity by 50 and 49%, PHF1/Tau5 ratio by 31 and 39%, and PHF1/CP27 ratio by 15 and 43%, respectively, after 4 weeks of treatment (*, p < 0.05, two-way analysis of variance, Bonferroni's post hoc test, n = 4 in each group). There were no differences in CP27, Tau5, or CP27/Tau5 ratios among different groups. D, quantified results of Sarkosyl-insoluble Tau detected by PHF1 antibody. The amount of Sarkosyl-insoluble Tau in antibody treated slices did not differ significantly from control slices. E, quantified results of the short term slice cultures treated with monoclonal antibodies for 2 h. Shown here is that neither mAbs had significant effects on the PHF1/Tau5 ratio in soluble and insoluble fractions. There was also no difference in PHF1 or Tau5 immunoreactivity in both fractions (data not shown).
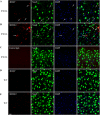
FIGURE 3.
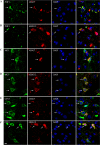
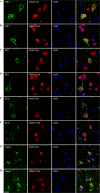
Co-staining of antibody or control IgG-treated brain slices with neuronal markers. Representative images of brains sections show the distribution of monoclonal antibodies or control IgG in neurons. A and B, labeled mAbs were widely detected in neurons in JNPL3 brain slices; C, labeled control IgG was not detected in JNPL3 slices; D and E, minimal uptake of mAbs was found in WT slices. Nuclei are stained blue with DAPI. Scale bar = 20 μm.
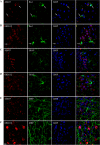
FIGURE 4.
Co-staining of antibody-treated JNPL3 brain slices with different cell markers. Representative images of brains sections show the distribution of monoclonal antibodies among different types of cells: A and B, Iba1 for microglia; C and D, glial fibrillary acidic protein for astrocytes; and E and F, myelin basic protein for oligodendrocytes. A small amount of mAbs was found in microglia, whereas no mAbs were found in astrocytes or oligodendrocytes. Nuclei are stained blue with DAPI. Scale bar = 20 μm.
FIGURE 5.
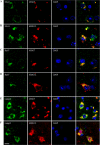
Co-staining of antibody-treated brain slices with different pathological Tau markers. A and B, 4E6G7 was highly co-localized with PHF1 signal. 6B2G12 exhibited a more diffused pattern in cytosol and was only partially co-localized with PHF1. C and D, 6B2G12 had better co-localization with MC1, which recognizes early abnormal conformation of Tau pathology, whereas 4E6G7 had a partial co-localization. E and F, both 4E6G7 and 6B2G12 showed a partial co-localization with CP13, which recognizes Tau Ser(P)-202. Arrows indicate colocalization. Nuclei are stained blue with DAPI. Scale bar = 10 μm.
FIGURE 6.
Co-staining of antibody-treated brain slices with endosome and lysosome markers. Representative images show the co-localizations between the monoclonal antibodies and EEA1 (A and B), an early endosome marker; Rab7, a late endosome/lysosome marker (C and D); and Lamp2, a lysosome marker (E and F). Note that 4E6G7 was highly co-localized with EEA1, Rab7, and Lamp2 in the perinuclear region. The more diffused localization of 6B2G12 only partially co-localized with these markers. Nuclei are stained blue with DAPI. Scale bar = 10 μm.
FIGURE 7.
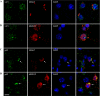
Co-staining of antibody-treated brain slices with autophagy markers. A and B, the monoclonal antibodies had little co-localization with LC3. C and D, the monoclonal antibodies showed a partial co-localization with p62. Arrows indicate colocalization between antibody and p62. Nuclei are stained blue with DAPI. Scale bar = 10 μm.
FIGURE 8.
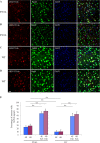
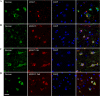
Co-staining of Fab fragment treated wild-type (WT) and transgenic (P301L) brain slices with neuronal markers. A–D, representative images of brains sections show the distribution of Fab fragments in neurons: A and B, transgenic; C and D, wild-type. The majority of Fab fragments were detected in neurons. E, quantified data shows the percentage of neurons that are positive for antibodies (4E and 6B) or Fab fragments (4E Fab and 6B Fab) in the cortical region of treated brain slices from P301L and WT mice. Fab fragments were taken up by a significantly larger portion of neurons in both P301L and WT slices, which were not different between the two groups (**, p < 0.01, t test. n = 3 mice in each group). Nuclei are stained blue with DAPI. 4E, 4E6G7; 6B, 6B2G12. Scale bar = 20 μm.
FIGURE 9.
Co-staining of Fab fragment-treated brain slices with pathological tau markers and endosome and lysosome markers. Fab fragments had a partial co-localization with pathological Tau markers PHF1 (A and B) and MC1 (C and D). Note that many neurons containing Fab fragments are negative for Tau pathology. Fab fragments co-localized infrequently with early endosomes (EEA1), but showed a partial co-localization with lysosomes (Lamp2) (E–H). Nuclei are stained blue with DAPI. Scale bar = 10 μm.
FIGURE 10.
Uptake of dextran, a bulk endocytosis marker, together with mAbs or Fab fragments. A and B, the mAbs co-localized partially with dextran. C and D, the Fab fragments showed a higher co-localization with dextran than mAbs. Nuclei are stained blue with DAPI. Scale bar = 20 μm.
Similar articles
- Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and is a prerequisite for acute tau protein clearance.
Congdon EE, Gu J, Sait HB, Sigurdsson EM. Congdon EE, et al. J Biol Chem. 2013 Dec 6;288(49):35452-65. doi: 10.1074/jbc.M113.491001. Epub 2013 Oct 25. J Biol Chem. 2013. PMID: 24163366 Free PMC article. - Conformation-selective tau monoclonal antibodies inhibit tau pathology in primary neurons and a mouse model of Alzheimer's disease.
Gibbons GS, Kim SJ, Wu Q, Riddle DM, Leight SN, Changolkar L, Xu H, Meymand ES, O'Reilly M, Zhang B, Brunden KR, Trojanowski JQ, Lee VMY. Gibbons GS, et al. Mol Neurodegener. 2020 Nov 4;15(1):64. doi: 10.1186/s13024-020-00404-5. Mol Neurodegener. 2020. PMID: 33148293 Free PMC article. - Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models.
Sankaranarayanan S, Barten DM, Vana L, Devidze N, Yang L, Cadelina G, Hoque N, DeCarr L, Keenan S, Lin A, Cao Y, Snyder B, Zhang B, Nitla M, Hirschfeld G, Barrezueta N, Polson C, Wes P, Rangan VS, Cacace A, Albright CF, Meredith J Jr, Trojanowski JQ, Lee VM, Brunden KR, Ahlijanian M. Sankaranarayanan S, et al. PLoS One. 2015 May 1;10(5):e0125614. doi: 10.1371/journal.pone.0125614. eCollection 2015. PLoS One. 2015. PMID: 25933020 Free PMC article. - Tau Immunotherapy.
Sigurdsson EM. Sigurdsson EM. Neurodegener Dis. 2016;16(1-2):34-8. doi: 10.1159/000440842. Epub 2015 Nov 10. Neurodegener Dis. 2016. PMID: 26551002 Free PMC article. Review. - Immunotherapy targeting pathological tau protein in Alzheimer's disease and related tauopathies.
Sigurdsson EM. Sigurdsson EM. J Alzheimers Dis. 2008 Oct;15(2):157-68. doi: 10.3233/jad-2008-15202. J Alzheimers Dis. 2008. PMID: 18953105 Free PMC article. Review.
Cited by
- Tau immunotherapy is associated with glial responses in FTLD-tau.
Kim B, Mikytuck B, Suh E, Gibbons GS, Van Deerlin VM, Vaishnavi SN, Spindler MA, Massimo L, Grossman M, Trojanowski JQ, Irwin DJ, Lee EB. Kim B, et al. Acta Neuropathol. 2021 Aug;142(2):243-257. doi: 10.1007/s00401-021-02318-y. Epub 2021 May 5. Acta Neuropathol. 2021. PMID: 33950293 Free PMC article. - Intratarget Microdosing for Deep Phenotyping of Multiple Drug Effects in the Live Brain.
Kim J, Ahn SW, Deans K, Thompson D, Ferland B, Divakar P, Dominas C, Jonas O. Kim J, et al. Front Bioeng Biotechnol. 2022 Mar 18;10:855755. doi: 10.3389/fbioe.2022.855755. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35372313 Free PMC article. - Development of brain-penetrable antibody radioligands for in vivo PET imaging of amyloid-β and tau.
Banka V, Kelleher A, Sehlin D, Hultqvist G, Sigurdsson EM, Syvänen S, Ding YS. Banka V, et al. Front Nucl Med. 2023;3:1173693. doi: 10.3389/fnume.2023.1173693. Epub 2023 May 4. Front Nucl Med. 2023. PMID: 37680310 Free PMC article. - Organotypic Hippocampal Slice Cultures from Adult Tauopathy Mice and Theragnostic Evaluation of Nanomaterial Phospho-TAU Antibody-Conjugates.
Kemppainen S, Huber N, Willman RM, Zamora A, Mäkinen P, Martiskainen H, Takalo M, Haapasalo A, Sobrino T, González Gómez MA, Piñeiro Y, Rivas J, Himmelreich U, Hiltunen M. Kemppainen S, et al. Cells. 2023 May 18;12(10):1422. doi: 10.3390/cells12101422. Cells. 2023. PMID: 37408256 Free PMC article. - Tau Filament Self-Assembly and Structure: Tau as a Therapeutic Target.
Oakley SS, Maina MB, Marshall KE, Al-Hilaly YK, Harrington CR, Wischik CM, Serpell LC. Oakley SS, et al. Front Neurol. 2020 Nov 12;11:590754. doi: 10.3389/fneur.2020.590754. eCollection 2020. Front Neurol. 2020. PMID: 33281730 Free PMC article. Review.
References
- Holmes C., Boche D., Wilkinson D., Yadegarfar G., Hopkins V., Bayer A., Jones R. W., Bullock R., Love S., Neal J. W., Zotova E., Nicoll J. A. (2008) Long-term effects of Aβ42 immunisation in Alzheimer's disease. Follow-up of a randomised, placebo-controlled phase I trial. Lancet 372, 216–223 - PubMed
- Siemers E. R., Friedrich S., Dean R. A., Gonzales C. R., Farlow M. R., Paul S. M., Demattos R. B. (2010) Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid β monoclonal antibody in subjects with Alzheimer disease. Clin. Neuropharmacol. 33, 67–73 - PubMed
- Farlow M., Arnold S. E., van Dyck C. H., Aisen P. S., Snider B. J., Porsteinsson A. P., Friedrich S., Dean R. A., Gonzales C., Sethuraman G., DeMattos R. B., Mohs R., Paul S. M., Siemers E. R. (2012) Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement. 8, 261–271 - PubMed
- Panza F., Frisardi V., Imbimbo B. P., Seripa D., Paris F., Santamato A., D'Onofrio G., Logroscino G., Pilotto A., Solfrizzi V. (2011) Anti-β-amyloid immunotherapy for Alzheimer's disease. Focus on bapineuzumab. Curr. Alzheimer Res. 8, 808–817 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG032611/AG/NIA NIH HHS/United States
- R01 AG020197/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- AG020197/AG/NIA NIH HHS/United States
- NS077239/NS/NINDS NIH HHS/United States
- R01 NS077239/NS/NINDS NIH HHS/United States
- AG032611/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical