Stimulation of mTORC1 with L-leucine rescues defects associated with Roberts syndrome - PubMed (original) (raw)
Stimulation of mTORC1 with L-leucine rescues defects associated with Roberts syndrome
Baoshan Xu et al. PLoS Genet. 2013.
Abstract
Roberts syndrome (RBS) is a human disease characterized by defects in limb and craniofacial development and growth and mental retardation. RBS is caused by mutations in ESCO2, a gene which encodes an acetyltransferase for the cohesin complex. While the essential role of the cohesin complex in chromosome segregation has been well characterized, it plays additional roles in DNA damage repair, chromosome condensation, and gene expression. The developmental phenotypes of Roberts syndrome and other cohesinopathies suggest that gene expression is impaired during embryogenesis. It was previously reported that ribosomal RNA production and protein translation were impaired in immortalized RBS cells. It was speculated that cohesin binding at the rDNA was important for nucleolar form and function. We have explored the hypothesis that reduced ribosome function contributes to RBS in zebrafish models and human cells. Two key pathways that sense cellular stress are the p53 and mTOR pathways. We report that mTOR signaling is inhibited in human RBS cells based on the reduced phosphorylation of the downstream effectors S6K1, S6 and 4EBP1, and this correlates with p53 activation. Nucleoli, the sites of ribosome production, are highly fragmented in RBS cells. We tested the effect of inhibiting p53 or stimulating mTOR in RBS cells. The rescue provided by mTOR activation was more significant, with activation rescuing both cell division and cell death. To study this cohesinopathy in a whole animal model we used ESCO2-mutant and morphant zebrafish embryos, which have developmental defects mimicking RBS. Consistent with RBS patient cells, the ESCO2 mutant embryos show p53 activation and inhibition of the TOR pathway. Stimulation of the TOR pathway with L-leucine rescued many developmental defects of ESCO2-mutant embryos. Our data support the idea that RBS can be attributed in part to defects in ribosome biogenesis, and stimulation of the TOR pathway has therapeutic potential.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
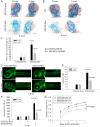
Figure 1. Human RBS cells showed severely slow proliferation and decreased protein translation.
(A). Immortalized WT, RBS, and corrected cells were seeded into 6-well plates with 0.5×105 cells/mL. After 8 days in culture, immortalized RBS cells showed very poor proliferation, compared with wild type (WT) and ESCO2-corrected (Cor) cells (P<0.01). Inhibition of p53, by Pifα (10 µM) incubation, partially rescued proliferation of RBS cells (P<0.05, 2-way ANOVA). (B). By FACScan analysis, RBS cells accumulated in G2/M phase. (C). Untransformed RBS and normal human skin fibroblasts (HSF) and amniotic fluid-derived cells (AFC) were grown in DMEM plus 15% FBS. 3H-uridine (5 µCi) was incubated with 106 cells from each group for two hours. Total RNA was isolated with TriZol reagent (Invitrogen, U.S.A) and the concentration of each RNA sample was measured by OD260/280. 1 µg of each sample was counted in a Beckman LS 6500 multipurpose scintillation counter to determine the amount of 3H-uridine incorporated. Four independent cultures were labeled to derive the standard deviation. Significance relative to normal cells was calculated using an unpaired t test. P = 0.0039, HSF ESCO2-Mut vs HSF ESCO2-WT; P = 0.000017, AFC ESCO2-Mut vs AFC ESCO2-WT. (D). Equal numbers of untransformed RBS and normal HSF and AFC cells were grown in DMEM plus 15% FBS. Cells were washed in PBS twice, switched to 3 mL Met/Cys-free Dulbecco's modied Eagle's medium containing 10 µM MG-132, a proteasome inhibitor, and pulsed with 30 µCi of 35S-methionine for 4 hrs. Cells were lysed in RIPA buffer and proteins were precipitated by the addition of hot 10% TCA. After centrifugation, the precipitate was washed twice in acetone. The precipitate was dissolved in 100 µL of 1% SDS and heated at 95°C for 10 min. An aliquot of the SDS extract was counted in Esoscint for 35S radioactivity in a liquid scintillation spectrometer to determine the amount of 35S-methionine incorporated into proteins. P = 0.00086, HSF ESCO2-Mut vs HSF ESCO2-WT; P = 0.0005, AFC ESCO2-Mut vs AFC ESCO2-WT.
Figure 2. The p53 pathway is upregulated in RBS cells.
(A–D). Western blot analysis showing p53, Mdm2, p21 and p27 were markedly activated in both immortalized (A–B) and untransformed RBS cells (C–D). Re-introduction of ESCO2 only partly reduced p53 activation in the immortalized RBS cells (A–B). The relative ratio of protein expression was quantified with ImageQuant TL software in (B, D). (E). Cell death was quantified by counting the number of trypan blue positive cells. Pifα incubation did not rescue cell death in human immortalized RBS cells.
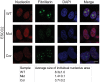
Figure 3. Nucleolar organization was severely disrupted in the immortalized RBS cells.
WT, RBS, and corrected RBS cells were immunostained with antibodies to the nucleolar components nucleolin and fibrillarin, and imaged with confocal microscopy. Scale bar = 10 µm. DNA was stained with DAPI. The average size (µm2) of individual nucleoli was measured in the fibrillarin stained sample using Image J software. About 20 cells were quantified for each sample.
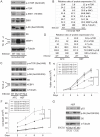
Figure 4. ESCO2 mutation is associated with mTOR inhibition in RBS cells.
(A–D). Phosphorylation of S6K1/S6 and 4EBP1-γ subunit ratio divided by α/β subunit was downregulated in the human immortalized RBS cells (A–B) and primary cells (C–D), as measured by Western blot analysis. The results are representative of three independent experiments. Total levels of S6, S6K1, and tubulin serve as loading controls. The relative ratios of protein expression were quantitated with ImageQuant TL software in (B, D). (E). L-Leu application partially improved poor proliferation of immortalized RBS cells. Each plot represents the average ± SEM of the ratio of the measurement of the indicated cell number, as calculated for three independent samples for 2-way ANOVA statistical analysis. P<0.01, HSF ESCO2-Mut with 10 mM D-Leu vs HSF ESCO2-WT with10 mM D-Leu; P<0.05, HSF ESCO2-Mut with 10 mM D-Leu vs HSF ESCO2-Mut with 10 mM L-Leu. (F). Elevated levels of cell death were partially suppressed by L-Leu supplementation. P<0.01, HSF ESCO2-Mut with 10 mM D-Leu vs HSF ESCO2-WT with 10 mM D-Leu; P<0.05, HSF ESCO2-Mut with 10 mM L-Leu vs HSF ESCO2-Mut with 10 mM D-Leu. (G). L-Leu, but not D-Leu, partially rescued the phosphorylated form of S6 in primary RBS fibroblasts. p53 levels were not rescued by treatment with L-Leu.
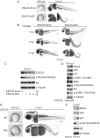
Figure 5. Reduced ESCO2 function is associated with mTOR inhibition, p53 activation, and dramatic developmental phenotypes in zebrafish.
Total levels of S6, S6K1, and tubulin serve as loading controls. (A). Embryos were microinjected with 4 ng ESCO2-5mismatched morpholino (ESCO2-5mis) or ESCO2-ATG morpholino (ESCO2-MO) and photographed at 24 hours post fertilization (h.p.f.) and 3 days post fertilization (d.p.f.). Scale bar = 200 µm. (B). Embryos were microinjected with 1, 2, or 4 ng ESCO2-5mis or ESCO2-MO and photographed at 3 d.p.f. Scale bar = 200 µm. (C). Embryos were microinjected with 4 ng ESCO2-5mis or 1, 2, or 4 ng ESCO2-MO to test the effect of dosage on phophorylation of S6 by Western blot at 2 d.p.f. (D). ESCO2 morphant embryos (2 ng) show inhibition of the TOR pathway and accompanying activation of p53 at 24 h.p.f.. (E). ESCO2-transgenic mutant zebrafish embryos show gross developmental abnormalities compared with WT embryos at 24 h.p.f. and 3 d.p.f.. Bar = 200 µm. (F). ESCO2 mutant embryos show reduced S6 phosphorylation and upregulation of p53, Mdm2, and p27 by Western blot analysis.
Figure 6. L-leucine partially improved developmental deficiencies of ESCO2 depleted embryos in a TOR pathway-dependent manner.
(A). Embryos (1–2 cells) were injected with ESCO2-5mis or ESCO2-MO (4 ng) and immediately separated for D-Leu or L-Leu incubation (10 mM) for 2 d.p.f. L-Leu supplement partially rescued development of ESCO2-morphant embryos at a gross level. Scale bar = 200 µm. Animals were categorized as mildly affected, severely affected, or dead to further quantify the rescue. (B). The number of severely malformed and lethal embryos was quantified for ESCO2-depleted embryos in the presence of D-Leu or L-Leu supplement at 4 days post fertilization. A total of 73-115 embryos were quantified per condition (ESCO2-5mis with D-Leu (n = 73), ESCO2-MO with D-Leu (n = 114), ESCO2-5mis with L-Leu (n = 97), and ESCO2-MO with L-Leu (n = 115)). (C). Embryos (1–2 cells) were injected with ESCO2-MO (4 ng) and immediately transferred to L-Leu incubation at different concentrations. L-Leu supplement ameliorated the developmental defects of ESCO2 morphant embryos in a dosage-dependent manner. While the image is representative, about 20 embryos were analyzed per group. Bar = 200 µm. (D). Embryos (1–2 cells) were injected with ESCO2-5mis or ESCO2-MO (4 ng) and immediately separated into D-Leu or L-Leu incubation (3 mM) for 24 h.p.f.. By Western blot analysis, L-Leu supplement partially rescued phosphorylation of S6 in the ESCO2-MO embryos. S6 and tubulin serve as loading controls. Each sample contains ∼100 embryos. (E). Embryos (1–2 cells) were injected with ESCO2-5mis or ESCO2-MO (4 ng), and immediately separated into D-Leu or L-Leu incubation (10 mM) for 3 d.p.f., in the presence or absence of 200 nM rapamycin. While the image is representative, about 15 embryos were analyzed per group. Rapamycin curtails L-Leu rescue of ESCO2-morphants, and enhances malformation of ESCO2-depleted embryos. Bar = 200 µm. (F). WT and ESCO2 mutant embryos (1–2 cells) were incubated with 10 mM D-Leu or L-Leu for 4 d.p.f., and photographed. L-Leu treatment partially rescued development of ESCO2 mutant embryos. While the image is representative, about 10 embryos were analyzed per group. Bar = 200 µm. (G). WT and ESCO2 mutant embryos (1–2 cells) were incubated with 10 mM D-Leu or L-Leu for 2 d.p.f. Western blotting shows L-Leu treatment partially restored phosphorylation of S6 in ESCO2 mutant embryos, but p53 elevation persists. S6 and tubulin serve as loading controls.
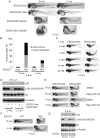
Figure 7. L-leucine rescues many aspects of development in ESCO2-depleted embryos.
(A). Embryos (1–2 cells) were injected with ESCO2-5mis or ESCO2-MO (4 ng), and immediately separated into D-Leu or L-Leu incubation (10 mM). After 5 d.p.f., the embryos were stained with Alcian blue to detect cartilage development. Scale bar = 200 µm. While the image is representative, about 15 embryos were analyzed per group. (B). WT or ESCO2 mutant embryos were treated with D-Leu or L-Leu (10 mM). After 5 d.p.f., the embryos were stained with Alcian blue to detect cartilage development. While the image is representative, about 10 embryos were analyzed per group. L-leucine partially rescued head size and cartilage formation in (A) and (B). (C). Cranial development was quantified for the data in (A) using the sum of the pq (palatoquadrate) cartilage and mc (Meckel's cartilage) divided by cranial length, as indicated with lines in (B). The measurement was done on 3 embryos per group. P<0.01, ESCO2-MO with D-Leu treatment vs ESCO2-5mis with D-Leu treatment; P<0.05, ESCO2-MO with L-Leu treatment vs ESCO2-MO with D-Leu treatment. (D). Embryos (1–2 cells) were injected with ESCO2-5mis or ESCO2-MO (2 ng), and immediately separated for D-Leu or L-Leu incubation (10 mM). After 2 d.p.f., the embryos were stained with acridine orange to detect apoptotic cells. Scale bar = 200 µm. (E). The number of apoptotic cells was quantified. P<0.0001, ESCO2-MO with D-Leu treatment vs ESCO2-5mis with D-Leu treatment; P = 0.0006, ESCO2-MO with L-Leu treatment vs ESCO2-MO with D-Leu treatment. (F). Embryos (1–2 cells) were treated as in (A). After 2 d.p.f., single cell suspensions of 10 embryos were generated in triplicate by mashing and filtering in PBS/10% NCS through cell strainers (100 µm, BD Falcon). Cells were resuspended in PBS/10% NCS and suspensions used in Caspase-Glo 3/7 Assays (Promega) according to the manufacturer's instructions. Luminescence was measured after 75 minutes on a multi-detection microplate reader. L-Leu treatment suppressed caspase 3/7 activation in the ESCO2-MO embryos. P<0.0001, ESCO2-5mis with D-Leu vs ESCO2-MO with D-Leu; P<0.0001, ESCO2-MO with L-Leu vs ESCO2-MO with D-Leu. (G). Embryos (1–2 cells) were treated as in (A). Embryo length was measured every day up to 4 d.p.f. with a mini-ruler under a Leica Stereoscope for ESCO2-MO and 5-mismatched controls. L-Leu treated ESCO2 morphants had a significantly longer body length compared with ESCO2 morphants treated with D-Leu (P<0.0001, 2-way ANOVA). The measurements were performed from head to tail for 10 embryos per group.
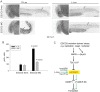
Figure 8. ESCO2 depletion in zebrafish is associated with an increase in phospho-H3 staining, and L-leucine partially rescues the increase.
(A). Embryos (1–2 cells) were injected with ESCO2-5mis or ESCO2-MO (4 ng), and immediately separated for D-Leu or L-Leu incubation (10 mM). At 24 h.p.f., cells were immunostained with anti-phospho-Histone H3 (pH3) antibody to detect mitotic cells. Scale bar = 200 µm. (B). The number of cells in mitosis was quantified for 5 embryos per group. P<0.0001, ESCO2-MO with D-Leu treatment vs ESCO2-5mis with D-Leu treatment; P<0.0001, ESCO2-MO with D-Leu treatment vs ESCO2-MO with L-Leu treatment. (C). A working model for the pathways involved in RBS is presented. Due to mutation in ESCO2, significant intracellular stress occurs due to defects in DNA replication, repair, and rDNA processes. This stress is detected by AMPK which can signal the activation of p53 , and the phosphorylation of TSC2. The phosphorylation of TSC2 will act to inhibit mTORC1 and downstream effectors such as 4EBP1, S6, and S6 kinase with the ultimate effect being the inhibition of translation. With the addition of L-leucine (green arrows), the leucyl tRNA synthetase will collaborate with the Rag GTPase to activate mTORC1, partially rescuing translation.
Similar articles
- Improved transcription and translation with L-leucine stimulation of mTORC1 in Roberts syndrome.
Xu B, Gogol M, Gaudenz K, Gerton JL. Xu B, et al. BMC Genomics. 2016 Jan 5;17:25. doi: 10.1186/s12864-015-2354-y. BMC Genomics. 2016. PMID: 26729373 Free PMC article. - A zebrafish model of Roberts syndrome reveals that Esco2 depletion interferes with development by disrupting the cell cycle.
Mönnich M, Kuriger Z, Print CG, Horsfield JA. Mönnich M, et al. PLoS One. 2011;6(5):e20051. doi: 10.1371/journal.pone.0020051. Epub 2011 May 26. PLoS One. 2011. PMID: 21637801 Free PMC article. - An ever-changing landscape in Roberts syndrome biology: Implications for macromolecular damage.
Mfarej MG, Skibbens RV. Mfarej MG, et al. PLoS Genet. 2020 Dec 31;16(12):e1009219. doi: 10.1371/journal.pgen.1009219. eCollection 2020 Dec. PLoS Genet. 2020. PMID: 33382686 Free PMC article. Review. - Cohesin proteins promote ribosomal RNA production and protein translation in yeast and human cells.
Bose T, Lee KK, Lu S, Xu B, Harris B, Slaughter B, Unruh J, Garrett A, McDowell W, Box A, Li H, Peak A, Ramachandran S, Seidel C, Gerton JL. Bose T, et al. PLoS Genet. 2012;8(6):e1002749. doi: 10.1371/journal.pgen.1002749. Epub 2012 Jun 14. PLoS Genet. 2012. PMID: 22719263 Free PMC article. - The non-redundant function of cohesin acetyltransferase Esco2: some answers and new questions.
Whelan G, Kreidl E, Peters JM, Eichele G. Whelan G, et al. Nucleus. 2012 Jul 1;3(4):330-4. doi: 10.4161/nucl.20440. Epub 2012 May 22. Nucleus. 2012. PMID: 22614755 Review.
Cited by
- Knockdown of AMPKα2 Promotes Pulmonary Arterial Smooth Muscle Cells Proliferation via mTOR/Skp2/p27(Kip1) Signaling Pathway.
Ke R, Liu L, Zhu Y, Li S, Xie X, Li F, Song Y, Yang L, Gao L, Li M. Ke R, et al. Int J Mol Sci. 2016 May 31;17(6):844. doi: 10.3390/ijms17060844. Int J Mol Sci. 2016. PMID: 27258250 Free PMC article. - Zebrafish raptor mutation inhibits the activity of mTORC1, inducing craniofacial defects due to autophagy-induced neural crest cell death.
Tucker SK, Ghosal R, Swartz ME, Zhang S, Eberhart JK. Tucker SK, et al. Development. 2024 Mar 15;151(6):dev202216. doi: 10.1242/dev.202216. Epub 2024 Mar 21. Development. 2024. PMID: 38512806 - Zebrafish Models for Human Skeletal Disorders.
Marí-Beffa M, Mesa-Román AB, Duran I. Marí-Beffa M, et al. Front Genet. 2021 Aug 5;12:675331. doi: 10.3389/fgene.2021.675331. eCollection 2021. Front Genet. 2021. PMID: 34490030 Free PMC article. Review. - Ribosomopathies: Global process, tissue specific defects.
Yelick PC, Trainor PA. Yelick PC, et al. Rare Dis. 2015 Apr 1;3(1):e1025185. doi: 10.1080/21675511.2015.1025185. eCollection 2015. Rare Dis. 2015. PMID: 26442198 Free PMC article. Review. - Growth and the Environment of Schizosaccharomyces pombe.
Petersen J, Russell P. Petersen J, et al. Cold Spring Harb Protoc. 2016 Mar 1;2016(3):pdb.top079764. doi: 10.1101/pdb.top079764. Cold Spring Harb Protoc. 2016. PMID: 26933253 Free PMC article.
References
- Michaelis C, Ciosk R, Nasmyth K (1997) Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91: 35–45. - PubMed
- Xiong B, Gerton JL Regulators of the cohesin network. Annu Rev Biochem 79: 131–153. - PubMed
- Nasmyth K Cohesin: a catenase with separate entry and exit gates? Nat Cell Biol 13: 1170–1177. - PubMed
- Zhang J, Shi X, Li Y, Kim BJ, Jia J, et al. (2008) Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell 31: 143–151. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
This work was funded by the Stowers Institute for Medical Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous