Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation - PubMed (original) (raw)
Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation
Zhaohui Qian et al. PLoS One. 2013.
Abstract
Little is known about the biology of the emerging human group c betacoronavirus, Middle East Respiratory Syndrome coronavirus (MERS-CoV). Because coronavirus spike glycoproteins (S) mediate virus entry, affect viral host range, and elicit neutralizing antibodies, analyzing the functions of MERS-CoV S protein is a high research priority. MERS-CoV S on lentivirus pseudovirions mediated entry into a variety of cell types including embryo cells from New World Eptesicus fuscus bats. Surprisingly, a polyclonal antibody to the S protein of MHV, a group a murine betacoronavirus, cross-reacted in immunoblots with the S2 domain of group c MERS-CoV spike protein. MERS pseudovirions released from 293T cells contained only uncleaved S, and pseudovirus entry was blocked by lysosomotropic reagents NH4Cl and bafilomycin and inhibitors of cathepsin L. However, when MERS pseudovirions with uncleaved S protein were adsorbed at 4°C to Vero E6 cells, brief trypsin treatment at neutral pH triggered virus entry at the plasma membrane and syncytia formation. When 293T cells producing MERS pseudotypes co-expressed serine proteases TMPRSS-2 or -4, large syncytia formed at neutral pH, and the pseudovirions produced were non-infectious and deficient in S protein. These experiments show that if S protein on MERS pseudovirions is uncleaved, then viruses enter by endocytosis in a cathepsin L-dependent manner, but if MERS-CoV S is cleaved, either during virus maturation by serine proteases or on pseudovirions by trypsin in extracellular fluids, then viruses enter at the plasma membrane at neutral pH and cause massive syncytia formation even in cells that express little or no MERS-CoV receptor. Thus, whether MERS-CoV enters cells within endosomes or at the plasma membrane depends upon the host cell type and tissue, and is determined by the location of host proteases that cleave the viral spike glycoprotein and activate membrane fusion.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
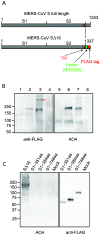
Figure 1. Incorporation of MERS-CoV SΔ16 protein into pseudovirions (A) Diagram of full length MERS-CoV S protein and MERS-CoV S∆16 protein with the C-terminal 16 amino acids substituted with a linker and FLAG tag.
S1, virus attachment domain; S2, membrane fusion domain; TM, transmembrane domain. (B) Detection of uncleaved MERS-CoV SΔ16 protein in cells and pseudovirions. Lanes 1 and 5, control pseudovirions with no spike; lanes 2 and 6, MERS pseudovirions; lanes 3 and 7, lysate of 293T cells producing MERS pseudovirions; lanes 4 and 8, lysate of 293T cells producing control pseudovirions with no spike. Lanes 1 to 4 were blotted with anti-FLAG; and lanes 5 to 8, with polyclonal goat antibody AO4 to MHV-A59 S protein. * indicates possible trimer of MERS-CoV S protein in pseudovirions. In the pseudovirions made in 293T cells, the ~200 kDa S protein is uncleaved. (C) Identification of the domain of MERS-CoV S protein that is recognized by polyclonal antibody to MHV spike protein. Truncated MERS-CoV S proteins with c-terminal FLAG tags were harvested from the medium over transfected cells, analyzed by SDS-PAGE, and then blotted with anti-FLAG or AO4 antibody. A lysate of 293T cells expressing full length codon-optimized MERS SΔ16 is shown in Lane 1 as a positive control for recognition of MERS S by AO4.
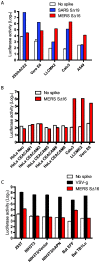
Figure 2. Entry into human, monkey and bat cells of pseudovirions with SARS-CoV, VSV or MERS-CoV glycoproteins.
(A) Entry of SARS (blue), MERS-CoV (red) or no spike control (white) pseudovirions into 293/hACE2 (293 cells stably expressing hACE2, the SARS-CoV receptor), Vero E6, LLCMK2, Calu3, and A549 cell lines. Pseudovirus entry was quantitated by luciferase activity at 40 hrs post inoculation (pi). (B) Entry of MERS-CoV (red) or no spike control (white) pseudovirions into HeLa cells expressing 6 different human CEACAM proteins, Calu3, LLCMK2 and Vero E6 cells. (C) Entry of VSV (black), MERS-CoV (red) or no spike control (white) pseudovirions into different cell lines: 293T, NIH3T3, NIH3T3/Vector (transfected with empty vector), NIH3T3/hAPN (stably expressing human APN), bat EFF (embryo cells from Eptesicus fuscus), and bat TB1Lu (lung cells from Tadarida brasiliensis).
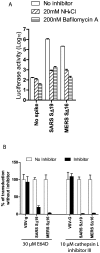
Figure 3. Inhibition of entry of MERS pseudovirions by lysosomotropic agents or cathepsin inhibitors.
(A) Entry into Vero E6 cells of pseudovirions with SARS S protein, uncleaved MERS-CoV S protein or no spike control in the presence of 20mM NH4Cl (bars with rising stripes), 200nM bafilomycin A (bars with horizontal stripes), or medium alone (no inhibitor control, white bars). (B) Effects of cathepsin inhibitors on entry of pseudovirions with uncleaved MERS-CoV S protein, SARS S or VSV-g protein. Broad spectrum cathepsin inhibitor, E64D, or cathepsin L-specific inhibitor, cathepsin L inhibitor III (black bars) reduced entry of MERS and SARS pseudovirions, but did not inhibit entry of VSV pseudovirions relative to no inhibitor controls (white bars). Virus entry was quantitated by luciferase activity at 40hr pi.
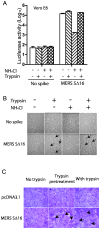
Figure 4. Trypsin activation of entry of MERS pseudovirions at the plasma membrane at neutral pH and MERS-CoV S-mediated syncytia formation.
(A) MERS pseudovirions or no spike control pseudovirions were adsorbed on Vero E6 cells at 4°C, then briefly treated with trypsin or medium alone, followed by trypsin inhibitor in the presence or absence of NH4Cl to inhibit acidification of endosomes. Pseudovirus entry was quantitated by luciferase activity at 40 hrs pi. (B) In the same experiment, addition of trypsin activated S-mediated formation of scattered syncytia (black arrows) in Vero E6 cells with adsorbed MERS pseudovirions both in the presence and absence of NH4Cl at 40hr pi. (C) 293T cells stably transfected with plasmid encoding MERS-CoV SΔ16 protein or with no spike were briefly pre-treated with trypsin at 4°C, then with soybean trypsin inhibitor and then co-cultured with Vero E6 cells for 20 hr, or were co-cultured with Vero E6 cells for 20 hr in the continuous presence of trypsin. Syncytia formation required expression on the Vero E6 cells of the MERS-CoV receptor as well as trypsin cleavage of MERS-CoV S protein, but did not require acid pH.
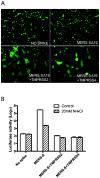
Figure 5. Effects of TMPRSS-2 and TMPRSS-4 on MERS-CoV S-mediated syncytia formation in 293T cells and MERS pseudovirus entry into Vero E6 cells.
(A) Co-expression in 293T cells of the transmembrane serine proteases TMPRSS-2 or TMPRSS-4 as well as MERS pseudovirions induced syncytia formation in 293T cells expressing the GFP reporter gene, visualized 40hr after transfection. (B) MERS pseudovirions or no spike control pseudovirions released from the cells in Figure 5A were inoculated onto Vero E6 cells in the presence (striped bars) or absence (white bars) of 20mM NH4Cl to inhibit acidification of endosomes.
Similar articles
- Middle East Respiratory Syndrome Coronavirus Spike Protein Is Not Activated Directly by Cellular Furin during Viral Entry into Target Cells.
Matsuyama S, Shirato K, Kawase M, Terada Y, Kawachi K, Fukushi S, Kamitani W. Matsuyama S, et al. J Virol. 2018 Sep 12;92(19):e00683-18. doi: 10.1128/JVI.00683-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021905 Free PMC article. - Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors.
Sisk JM, Frieman MB, Machamer CE. Sisk JM, et al. J Gen Virol. 2018 May;99(5):619-630. doi: 10.1099/jgv.0.001047. Epub 2018 Mar 20. J Gen Virol. 2018. PMID: 29557770 Free PMC article. - Ca2+ Ions Promote Fusion of Middle East Respiratory Syndrome Coronavirus with Host Cells and Increase Infectivity.
Straus MR, Tang T, Lai AL, Flegel A, Bidon M, Freed JH, Daniel S, Whittaker GR. Straus MR, et al. J Virol. 2020 Jun 16;94(13):e00426-20. doi: 10.1128/JVI.00426-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295925 Free PMC article. - MERS-CoV spike protein: Targets for vaccines and therapeutics.
Wang Q, Wong G, Lu G, Yan J, Gao GF. Wang Q, et al. Antiviral Res. 2016 Sep;133:165-77. doi: 10.1016/j.antiviral.2016.07.015. Epub 2016 Jul 26. Antiviral Res. 2016. PMID: 27468951 Free PMC article. Review. - Bat-to-human: spike features determining 'host jump' of coronaviruses SARS-CoV, MERS-CoV, and beyond.
Lu G, Wang Q, Gao GF. Lu G, et al. Trends Microbiol. 2015 Aug;23(8):468-78. doi: 10.1016/j.tim.2015.06.003. Epub 2015 Jul 21. Trends Microbiol. 2015. PMID: 26206723 Free PMC article. Review.
Cited by
- Leaving no stone unturned: Allosteric targeting of SARS-CoV-2 spike protein at putative druggable sites disrupts human angiotensin-converting enzyme interactions at the receptor binding domain.
Olotu FA, Omolabi KF, Soliman MES. Olotu FA, et al. Inform Med Unlocked. 2020;21:100451. doi: 10.1016/j.imu.2020.100451. Epub 2020 Oct 16. Inform Med Unlocked. 2020. PMID: 33083517 Free PMC article. - Lectins Are the Sparkle of Hope for Combating Coronaviruses and the Global COVID-19.
Abbas HS, Kotakonda M. Abbas HS, et al. Adv Pharm Bull. 2022 Mar;12(2):319-328. doi: 10.34172/apb.2022.030. Epub 2021 Mar 27. Adv Pharm Bull. 2022. PMID: 35620331 Free PMC article. Review. - Polymorphisms in dipeptidyl peptidase 4 reduce host cell entry of Middle East respiratory syndrome coronavirus.
Kleine-Weber H, Schroeder S, Krüger N, Prokscha A, Naim HY, Müller MA, Drosten C, Pöhlmann S, Hoffmann M. Kleine-Weber H, et al. Emerg Microbes Infect. 2020 Jan 21;9(1):155-168. doi: 10.1080/22221751.2020.1713705. eCollection 2020. Emerg Microbes Infect. 2020. PMID: 31964246 Free PMC article. - Canine respiratory coronavirus employs caveolin-1-mediated pathway for internalization to HRT-18G cells.
Szczepanski A, Owczarek K, Milewska A, Baster Z, Rajfur Z, Mitchell JA, Pyrc K. Szczepanski A, et al. Vet Res. 2018 Jul 3;49(1):55. doi: 10.1186/s13567-018-0551-9. Vet Res. 2018. PMID: 29970183 Free PMC article. - Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations.
Singh AK, Gupta R, Ghosh A, Misra A. Singh AK, et al. Diabetes Metab Syndr. 2020 Jul-Aug;14(4):303-310. doi: 10.1016/j.dsx.2020.04.004. Epub 2020 Apr 9. Diabetes Metab Syndr. 2020. PMID: 32298981 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources