Bone marrow deficiency of TRPC3 channel reduces early lesion burden and necrotic core of advanced plaques in a mouse model of atherosclerosis - PubMed (original) (raw)
Bone marrow deficiency of TRPC3 channel reduces early lesion burden and necrotic core of advanced plaques in a mouse model of atherosclerosis
Jean-Yves Tano et al. Cardiovasc Res. 2014.
Abstract
Aims: Macrophage apoptosis plays a determinant role in progression of atherosclerotic lesions. An important goal in atherosclerosis research is to identify new components of macrophage apoptosis that can eventually be exploited as molecular targets in strategies aimed at manipulating macrophage function in the lesion. In the previous work from our laboratory, we have shown that transient receptor potential canonical 3 (TRPC3) channel is an obligatory component of survival mechanisms in human and murine macrophages and that TRPC3-deficient non-polarized bone marrow-derived macrophages exhibit increased apoptosis, suggesting that in vivo TRPC3 might influence lesion development. In the present work, we used a bone marrow transplantation strategy as a first approach to examine the impact of macrophage deficiency of TRPC3 on early and advanced atherosclerotic lesions of Apoe(-/-) mice.
Methods and results: After 3 weeks of high-fat diet, lesions in mice transplanted with bone marrow from Trpc3(-/-) donors were smaller and with reduced cellularity than controls. Advanced lesions from these mice exhibited reduced necrotic core, less apoptotic macrophages, and increased collagen content and cap thickness. In vitro, TRPC3-deficient macrophages polarized to the M1 phenotype showed reduced apoptosis, whereas both M1 and M2 macrophages had increased efferocytic capacity.
Conclusions: Bone marrow deficiency of TRPC3 has a dual beneficial effect on lesion progression by reducing cellularity at early stages and necrosis in the advanced plaques. Our findings represent the first evidence for a role of a member of the TRPC family of cation channels in mechanisms associated with atherosclerosis.
Keywords: Atherosclerosis; Calcium channels; Macrophage apoptosis; TRPC3 channel.
Figures
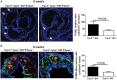
Figure 1
Aortic root sections from Trpc3+/+Apoe−/−BM→Apoe−/− or Trpc3−/−Apoe−/−BM→Apoe−/− mice on a 3-week high-fat diet were stained with haematoxylin–eosin (A), or Oil-Red-O (B) to evaluate lesion area and lipid content, respectively. Quantifications of mean stained areas are shown. Trpc3+/+Apoe−/−BM→Apoe−/− (n = 11–12), Trpc3−/−Apoe−/− BM→Apoe−/− (n = 13–14).
Figure 2
Aortic root sections from Trpc3+/+Apoe−/−BM→Apoe−/− or Trpc3−/−Apoe−/−BM→Apoe−/− mice on an 8-week high-fat diet were stained with haematoxylin–eosin (A), or Oil-Red-O (B). Quantifications of mean stained areas are shown. Trpc3+/+Apoe−/−BM→Apoe−/− (n = 13), Trpc3−/−Apoe−/− BM→Apoe−/− (n = 10–11).
Figure 3
Aortic root sections from Trpc3+/+Apoe−/−BM→Apoe−/− or Trpc3−/−Apoe−/−BM→Apoe−/− mice maintained on an 8-week high-fat diet were stained with Masson's trichrome to evaluate collagen content (blue) and necrosis. Representative sections are shown in (A). (B) The mean per cent collagen content relative to the lesion area. (C) Cap thickness, measured from largest necrotic cores (n = 32 for Trpc3+/+Apoe−/−; n = 21 for Trpc3−/−Apoe−/−) from 10 to 13 mice per group.
Figure 4
(A) Aortic root sections from Trpc3+/+Apoe−/−BM→Apoe−/− or Trpc3−/−Apoe−/−BM→Apoe−/− mice maintained on a high-fat diet for 8 weeks were stained for in situ TUNEL to detect apoptotic cells (arrows). In (B) representative merged images are shown of sections co-stained for macrophage (green, AIA31240), TUNEL, and DAPI. _P_-values were determined using the Mann–Whitney U test.
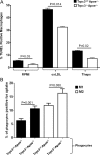
Figure 5
(A) Bone marrow-derived Trpc3+/+Apoe−/− or Trpc3−/−Apoe−/− macrophages differentiated to the M1 phenotype were incubated (24 h) in serum-free RPMI (RPMI) or RPMI containing oxidized-LDL (oxLDL, 50 µg/mL) or thapsigargin (1 µM), and processed for the TUNEL assay. ‘ns’: not statistically significant. Values are means ± SEM (n = 3). (B) Apoptotic non-polarized (M0) BMDMs from Trpc3+/+Apoe−/− mice were labelled with Calcein green and added to Trpc3+/+Apoe−/− or Trpc3−/−Apoe−/− M1 or M2 macrophages to evaluate efferocytosis. Shown are means ± SEM values (n = 3).
Similar articles
- Reduced Necrosis and Content of Apoptotic M1 Macrophages in Advanced Atherosclerotic Plaques of Mice With Macrophage-Specific Loss of Trpc3.
Solanki S, Dube PR, Birnbaumer L, Vazquez G. Solanki S, et al. Sci Rep. 2017 Feb 10;7:42526. doi: 10.1038/srep42526. Sci Rep. 2017. PMID: 28186192 Free PMC article. - Reduced endoplasmic reticulum stress-induced apoptosis and impaired unfolded protein response in TRPC3-deficient M1 macrophages.
Solanki S, Dube PR, Tano JY, Birnbaumer L, Vazquez G. Solanki S, et al. Am J Physiol Cell Physiol. 2014 Sep 15;307(6):C521-31. doi: 10.1152/ajpcell.00369.2013. Epub 2014 Jul 16. Am J Physiol Cell Physiol. 2014. PMID: 25031020 Free PMC article. - Reduced calcification and osteogenic features in advanced atherosclerotic plaques of mice with macrophage-specific loss of TRPC3.
Dube PR, Chikkamenahalli LL, Birnbaumer L, Vazquez G. Dube PR, et al. Atherosclerosis. 2018 Mar;270:199-204. doi: 10.1016/j.atherosclerosis.2017.12.025. Epub 2017 Dec 22. Atherosclerosis. 2018. PMID: 29290366 Free PMC article. - Macrophage function in atherosclerosis: potential roles of TRP channels.
Tano JY, Lee RH, Vazquez G. Tano JY, et al. Channels (Austin). 2012 May-Jun;6(3):141-8. doi: 10.4161/chan.20292. Channels (Austin). 2012. PMID: 22909953 Free PMC article. Review. - Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency.
Tabas I. Tabas I. Arterioscler Thromb Vasc Biol. 2005 Nov;25(11):2255-64. doi: 10.1161/01.ATV.0000184783.04864.9f. Epub 2005 Sep 1. Arterioscler Thromb Vasc Biol. 2005. PMID: 16141399 Review.
Cited by
- miR-26a inhibits atherosclerosis progression by targeting TRPC3.
Feng M, Xu D, Wang L. Feng M, et al. Cell Biosci. 2018 Jan 19;8:4. doi: 10.1186/s13578-018-0203-9. eCollection 2018. Cell Biosci. 2018. PMID: 29387339 Free PMC article. - Mechanosensing and Mechanosignal Transduction in Atherosclerosis.
Rahaman SG, Mahanty M, Mukherjee P, Dutta B, Rahaman SO. Rahaman SG, et al. Curr Atheroscler Rep. 2023 Oct;25(10):711-721. doi: 10.1007/s11883-023-01139-6. Epub 2023 Aug 24. Curr Atheroscler Rep. 2023. PMID: 37615786 Review. - Function of TRP channels in monocytes/macrophages.
Wu J, Li Z, Deng Y, Lu X, Luo C, Mu X, Zhang T, Liu Q, Tang S, Li J, An Q, Fan D, Xiang Y, Wu X, Hu Y, Du Q, Xu J, Xie R. Wu J, et al. Front Immunol. 2023 Jun 19;14:1187890. doi: 10.3389/fimmu.2023.1187890. eCollection 2023. Front Immunol. 2023. PMID: 37404813 Free PMC article. Review. - Endoplasmic reticulum stress: a novel mechanism and therapeutic target for cardiovascular diseases.
Liu MQ, Chen Z, Chen LX. Liu MQ, et al. Acta Pharmacol Sin. 2016 Apr;37(4):425-43. doi: 10.1038/aps.2015.145. Epub 2016 Feb 1. Acta Pharmacol Sin. 2016. PMID: 26838072 Free PMC article. Review. - Micro-RNAs and macrophage diversity in atherosclerosis: new players, new challenges…new opportunities for therapeutic intervention?
Vazquez G. Vazquez G. Biochem Biophys Rep. 2015 Sep 1;3:202-206. doi: 10.1016/j.bbrep.2015.08.009. Biochem Biophys Rep. 2015. PMID: 26457329 Free PMC article.
References
- Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, et al. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. - PubMed
- Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney J, James W. The mammalian TRPC cation channels. Biochim Biophys Acta Mol Cell Res. 2004;1742:21–36. - PubMed
- Tano J-Y, Smedlund K, Lee R, Abramowitz J, Birnbaumer L, Vazquez G. Impairment of survival signaling and efferocytosis in TRPC3-deficient macrophages. Biochem Biophys Res Commun. 2011;410:643–647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous