Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4 - PubMed (original) (raw)
Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4
Ekaterina Kurakevich et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Breast milk oligosaccharides shape the intestinal environment by affecting mucosal immunity and bacterial colonization. To clarify the role of milk oligosaccharide sialyl(α2,3)lactose (3SL) in intestinal physiology and disease, we investigated colitis development in Il10(-/-) mice exposed to normal or 3SL-deficient milk during lactation. Onset and progression of intestinal inflammation were delayed in Il10(-/-) mice deficient for the α2,3 sialyltransferase 4 (ST3GAL4) responsible for 3SL biosynthesis. The proinflammatory role of 3SL was confirmed by showing that oral supplementation of newborn Il10(-/-);St3gal4(-/-) mice with 3SL increased colitis severity. Conversely, fostering of newborn Il10(-/-) mice to lactating St3gal4(-/-) mothers reduced colitis severity. 3SL directly stimulated mesenteric lymph node CD11c(+) dendritic cells and induced production of cytokines required for expansion of TH1 and TH17 T cells. The stimulatory effect of 3SL was attenuated in Tlr4-deficient CD11c(+) cells, demonstrating that 3SL induces inflammation through Toll-like receptor 4 (TLR4) signaling. Thus, 3SL directly modulates mucosal immunity, which increases susceptibility to colitis.
Keywords: carbohydrate; glycan; mouse innate immunity; prebiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
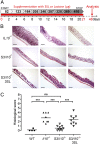
Fig. 1.
St3gal4 deficiency attenuates spontaneous intestinal inflammation in Il10 −/− mice. (A) Microscopic analysis of colon sections from 6- and 14-wk-old _Il10_−/− and S3/I10−/− mice, stained with H&E. Representative images from three independent experiments. (Scale bar, 200 μm.) (B) Histological score based on evaluation of morphological changes of epithelium and immune cell infiltration. Six- and 14-wk-old Il10 −/− and S3/I10−/− mice (n = 8–12) were analyzed. (C) Frequency of rectal prolapse in _Il10_−/− and S3/I10−/− mice. Il10 −/− mice (n = 46) and S3/I10−/− mice (n = 63) were monitored over a period of 26 wk. The graph represents percentage of mice with rectal prolapse from the total number mice at the age of 10–12 wk and 24–26 wk, respectively. WT, wild-type mice; S3/I10−/−, St3g4 −/− ; Il10 −/− mice; 6W, 6-wk-old mice; 14W, 14-wk-old mice.
Fig. 2.
Decreased leukocyte infiltration and inflammation in colons of St3gal4 −/− /Il10 −/− mice. (A) Flow cytometry analysis of lamina propria leukocytes (LPLs) isolated from a distal part of the colon of 6- and 14-wk-old Il10 −/− and S3/I10−/− mice (n = 5–7) or 6- to 8-wk-old WT controls. Data are presented as percentage of CD45+, CD4+, CD4+FoxP3+ (Treg), CD11c+, Ly6Chi, and Ly6G+ cells from all isolated cells. (B) Expression levels of cytokines in colons of 6- and 14-wk-old Il10 −/−, S3/I10−/− mice (n = 6–8) and WT control mice (n = 5–6) were determined by real-time PCR and normalized to GAPDH.
Fig. 3.
3SL supplementation aggravates colonic inflammation. S3/I10−/− mice were fed daily from birth until weaning (21 d) with 25 mM 3SL or lactose; control mice were fed with water. Mice were analyzed at the age of 6 wk (day 48). (A) Schematic representation of 3SL and lactose supplementation. (B) Representative microscopic images (n = 9) of colon sections from 6-wk-old _Il10_−/− and S3/I10−/− mice either with or without 3SL supplementation stained with hematoxylin and eosin. (Scale bar, 200 μm.) (C) Histological score based on evaluation of morphological changes of epithelium and immune cell infiltration from WT (n = 4), Il10 −/−, and S3/I10−/− mice (n = 8–9). WT, wild-type mice; S3/I10−/−, St3g4 −/− ; Il10 −/− mice; 3SL, sialyl(α2,3)lactose.
Fig. 4.
3SL directly stimulates dendritic cells (DCs). DCs were isolated from mesenteric lymph nodes of 6-wk-old WT, St3gal4 −/−, and Il10 −/− mice and purified with CD11c MicroBeads. (A) CD11c+ cells were stimulated for 14 h with 625 μM of 3SL, 6SL, or lactose (Lac). Stimulations with LPS (500 ng/mL) or PBS (buff.) were used as controls. Cell surface expression of CD40, CD80, and CD86 was analyzed by flow cytometry. (B) Measurement of secreted cytokines in the medium of stimulated CD11c+ cells. (C) Increase in Ly-6C+/CD11c+ cells in Il10 −/− mice during inflammation. Data are presented as percentage of CD11b+, CD103+, Ly-6C+, or CD8α+ cells from CD11c+ cells (n = 6). MFI, mean fluorescence intensity; 3SL, sialyl(α2,3)lactose; 6SL, sialyl(α2,6)lactose.
Fig. 5.
3SL feeding induces colon inflammation and 3SL is sensed by TLR4. Six-week-old Il10 −/− mice were treated with 3SL, lactose (Lac), or control (null) for 4 d and analyzed at day 5 (n = 6 per group). (A) Microscopic analysis of colon sections. (Scale bar, 200 μm.) (B) Histological score based on evaluation of morphological changes of epithelium and immune cell infiltration. (C) Lamina propria CD45+ cells are presented as percentage of all isolated cells. (D) Expression levels of cytokines in colons of mice fed with 3SL and lactose (Lac) for 4 d were determined by real-time PCR and normalized to GAPDH (n = 5). (E) CD11c+ cells from MLN of WT, Myd88 −/−, Tlr2 −/−, and Tlr4 −/− mice were stimulated with 625 μM of 3SL or 6SL for 14 h. Stimulation with LPS (500 ng/mL) or PBS (buffered) was used as controls. Cell surface expression of CD80, CD86, and CD40 on CD11c+/MHCII+ cells was analyzed by flow cytometry and quantified in two independent experiments (n = 4). MFI, mean fluorescence intensity; 3SL, sialyl(α2,3)lactose; 6SL, sialyl(α2,6)lactose; Unstim, nonstimulated cells.
Similar articles
- Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization.
Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, Hennet T. Fuhrer A, et al. J Exp Med. 2010 Dec 20;207(13):2843-54. doi: 10.1084/jem.20101098. Epub 2010 Nov 22. J Exp Med. 2010. PMID: 21098096 Free PMC article. - The role of milk sialyllactose in intestinal bacterial colonization.
Weiss GA, Hennet T. Weiss GA, et al. Adv Nutr. 2012 May 1;3(3):483S-8S. doi: 10.3945/an.111.001651. Adv Nutr. 2012. PMID: 22585928 Free PMC article. - IL-10 control of CD11c+ myeloid cells is essential to maintain immune homeostasis in the small and large intestine.
Girard-Madoux MJ, Ober-Blöbaum JL, Costes LM, Kel JM, Lindenbergh-Kortleve DJ, Brouwers-Haspels I, Heikema AP, Samsom JN, Clausen BE. Girard-Madoux MJ, et al. Oncotarget. 2016 May 31;7(22):32015-30. doi: 10.18632/oncotarget.8337. Oncotarget. 2016. PMID: 27027442 Free PMC article. - Decoding breast milk oligosaccharides.
Hennet T, Weiss A, Borsig L. Hennet T, et al. Swiss Med Wkly. 2014 Feb 19;144:w13927. doi: 10.4414/smw.2014.13927. Swiss Med Wkly. 2014. PMID: 24554478 Review. - Immune responses of TLR5(+) lamina propria dendritic cells in enterobacterial infection.
Uematsu S, Akira S. Uematsu S, et al. J Gastroenterol. 2009;44(8):803-11. doi: 10.1007/s00535-009-0094-y. Epub 2009 Jun 24. J Gastroenterol. 2009. PMID: 19547909 Review.
Cited by
- Food allergies: the basics.
Valenta R, Hochwallner H, Linhart B, Pahr S. Valenta R, et al. Gastroenterology. 2015 May;148(6):1120-31.e4. doi: 10.1053/j.gastro.2015.02.006. Epub 2015 Feb 11. Gastroenterology. 2015. PMID: 25680669 Free PMC article. Review. - Carbohydrate intake in the etiology of Crohn's disease and ulcerative colitis.
Chan SS, Luben R, van Schaik F, Oldenburg B, Bueno-de-Mesquita HB, Hallmans G, Karling P, Lindgren S, Grip O, Key T, Crowe FL, Bergmann MM, Overvad K, Palli D, Masala G, Khaw KT, Racine A, Carbonnel F, Boutron-Ruault MC, Olsen A, Tjonneland A, Kaaks R, Tumino R, Trichopoulou A, Hart AR. Chan SS, et al. Inflamm Bowel Dis. 2014 Nov;20(11):2013-21. doi: 10.1097/MIB.0000000000000168. Inflamm Bowel Dis. 2014. PMID: 25265262 Free PMC article. - Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation.
He Y, Lawlor NT, Newburg DS. He Y, et al. Adv Nutr. 2016 Jan 15;7(1):102-11. doi: 10.3945/an.115.010090. Print 2016 Jan. Adv Nutr. 2016. PMID: 26773018 Free PMC article. Review. - Molecular Dynamics Simulations Reveal the Conformational Transition of GH33 Sialidases.
Cao X, Yang X, Xiao M, Jiang X. Cao X, et al. Int J Mol Sci. 2023 Apr 6;24(7):6830. doi: 10.3390/ijms24076830. Int J Mol Sci. 2023. PMID: 37047800 Free PMC article. - Induction of human tolerogenic dendritic cells by 3'-sialyllactose via TLR4 is explained by LPS contamination.
Perdijk O, van Neerven RJJ, Meijer B, Savelkoul HFJ, Brugman S. Perdijk O, et al. Glycobiology. 2018 Mar 1;28(3):126-130. doi: 10.1093/glycob/cwx106. Glycobiology. 2018. PMID: 29281012 Free PMC article.
References
- Molodecky NA, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54, e42, quiz e30. - PubMed
- Chapman-Kiddell CA, Davies PS, Gillen L, Radford-Smith GL. Role of diet in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(1):137–151. - PubMed
- Gearry RB, Richardson AK, Frampton CM, Dodgshun AJ, Barclay ML. Population-based cases control study of inflammatory bowel disease risk factors. J Gastroenterol Hepatol. 2010;25(2):325–333. - PubMed
- Bode L. Human milk oligosaccharides: Prebiotics and beyond. Nutr Rev. 2009;67(Suppl 2):S183–S191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials