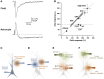How do astrocytes shape synaptic transmission? Insights from electrophysiology - PubMed (original) (raw)
Review
How do astrocytes shape synaptic transmission? Insights from electrophysiology
Glenn Dallérac et al. Front Cell Neurosci. 2013.
Abstract
A major breakthrough in neuroscience has been the realization in the last decades that the dogmatic view of astroglial cells as being merely fostering and buffering elements of the nervous system is simplistic. A wealth of investigations now shows that astrocytes actually participate in the control of synaptic transmission in an active manner. This was first hinted by the intimate contacts glial processes make with neurons, particularly at the synaptic level, and evidenced using electrophysiological and calcium imaging techniques. Calcium imaging has provided critical evidence demonstrating that astrocytic regulation of synaptic efficacy is not a passive phenomenon. However, given that cellular activation is not only represented by calcium signaling, it is also crucial to assess concomitant mechanisms. We and others have used electrophysiological techniques to simultaneously record neuronal and astrocytic activity, thus enabling the study of multiple ionic currents and in depth investigation of neuro-glial dialogues. In the current review, we focus on the input such approach has provided in the understanding of astrocyte-neuron interactions underlying control of synaptic efficacy.
Keywords: dual recordings; electrophysiology; glia; ionic channels; neuroglial interactions; neurons; plasticity; synapses.
Figures
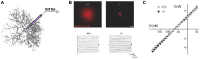
Figure 1
Recording of astroglial membrane properties. (A) Schematic representation of intracellular (whole cell patch clamp recording or sharp electrode) recording of an astrocyte. (B) Upper panel: dye coupling experiments show tens of coupled cells after patching of a single astrocyte with an intra-pipette solution containing sulforhodamine-B (red). Knockout mice for astroglial connexins (Cx30−/−Cx43fl/fl hGFAP-cre) exhibit a total absence of astrocytic gap junctional coupling. Lower panel: to determine astroglial membrane resistance in a whole cell patch clamp configuration, short incremental voltage pulses are imposed to the astroglial membrane clamped at −80 mV, and evoked currents are recorded. (C) Quantification of the current/voltage relationship (IV curve). Illustration depicting current responses recorded in a hippocampal astrocyte from wild type and astroglial connexins knockout (Cx30−/−Cx43fl/fl hGFAP-cre) animals. Both groups display a quasi-ohmic profile of the IV curve, with a similar slope, indicating a comparable membrane resistance. Adapted, with permission, from Pannasch et al. (2011) (B,C).
Figure 2
Recording of synaptically evoked GLT and K+ currents in astrocytes. Schematic representation of the experimental setting for simultaneous recording of extracellular fEPSP (EXTRA) and intracellular astrocytic currents (INTRA) (A) and of the main channels and transporters involved in the synaptically evoked astroglial currents at the tripartite synapse (B). Simultaneous recordings of hippocampal fEPSP and astroglial currents evoked synaptically by single stimulation in basal conditions, after application of the ionotropic glutamate receptor blocker kynurenic acid, and further application of the GLT antagonist TBOA. To isolate excitatory currents, the GABAA receptor blocker picrotoxin is present throughout the experiment (C). Note that the initial fast outward current component in the presence of picrotoxin reflects fEPSP generated by adjacent pyramidal cells. Astroglial K+ currents are isolated by subtracting currents remaining after kynurenic acid application (2) to total evoked currents (1). Astroglial GLT currents are then isolated by further subtraction of the currents remaining after TBOA application (3) (D). Adapted, with permission from Pannasch et al. (2012b) (C,D).
Figure 3
High potassium permeability of glial cells is mediated by Kir4.1 channels. (A) Schematic illustration of simultaneous recordings of extracellular K+ concentration (K+) and glial membrane potential (INTRA). (B) In vivo simultaneous recordings of extracellular K+ concentrations and glial membrane potential fluctuations during long lasting stimulations (10 Hz, 30 s). Glial membranes behave as K+ electrodes, reflecting their high K+ permeability. Abbreviation for Nernst equation: R, universal gas constant; T, temperature; F, Faraday constant; EK, K+ equilibrium potential; Vm, membrane potential; [K+]o, extracellular K+ concentration; [K+]i, intracellular K+ concentration. (C) Similar protocol applied in a Kir4.1 knockout animal. Glial membrane potential does not follow K+ fluctuations, indicating a strong loss of K+ conductances. A small and delayed depolarization, which is not associated with K+ increase, could, however, be observed in response to long lasting stimulations only. Adapted, with permission, from Chever et al. (2010) (B,C).
Figure 4
Dual electrophysiological recording of neuronal and astroglial activities. (A) Example showing that the extracellular field response corresponds to the initial outward signal recorded through an astrocytic patch pipette in the voltage clamp configuration. (B) Correlation between field and glial responses to various manipulations shows a linear relationship that falls in the line of identity. Adeno, adenosine application; PPF, paired pulse facilitation; CPT, A1 receptor antagonist CPT, High stim, high stimulation intensity. (C–F) Possible experimental arrangements for simultaneously monitoring neuronal and astroglial activities. (C) Double whole cell patch clamp experiments give access to intracellular currents for both astrocytes and neurons. (D) Typical electrode placement for monitoring fEPSP in parallel to astrocytic currents. (E) Such electrode placement can be extended to include an internal control pathway in the experimental set-up. (F) Monitoring the fEPSP through the astrocytic glass pipette greatly simplifies such arrangements. Adapted, with permission, from Diamond et al. (1998) (A), Lüscher et al. (1998) (B), and Henneberger and Rusakov (2012) (C–F).
Figure 5
Interfering with astrocytes influences neuronal activity. (A,B) Infusion of BAPTA in the astrocytic network decreases synaptic efficacy in minimal stimulation conditions. (A) Experimental arrangement showing whole cell patch clamp of an astrocyte (PipAstro) present in the dendritic tree of the recorded granule cell and 20–30 μm away from the stimulating pipette (Pipstim). The intra-pipette solution for patch clamp contains the Ca2+ chelator BAPTA. (B) Failure rate changes in granule cells after breaking the cell membrane and dialyzing the astrocyte with the intra-pipette solution containing BAPTA. (C–E) Measure of astrocytic glutamate release using the sniffer patch technique. (C) Schematic illustration of the sniffer-patch. The left pipette (yellow) is used for pressure application of the PAR-1 agonist TFLLR. This stimulation results in glutamate release (red cloud) producing a measureable inward current in the HEK293T sensor cell (green) expressing GluR1-L497Y containing AMPA receptors (red). (D) Images for sniffer patch. DIC image (upper left): two cells with two glass pipettes. GFP image (upper right): sensor cell expressing GluR1-L497Y and GFP. Pseudocolor images: Fura-2 loaded astrocyte (source cell) and sensor cells before (lower left) and after stimulation (lower right). Yellow arrow: increased Ca2+ in astrocyte. (E) Representative traces recorded from the sniffer-patch technique. Blue trace: Ca2+ transient recorded from astrocyte. Green trace: whole cell current recorded from sensor cell (voltage clamped at −70 mV) upon TFLLR pressure application. Diamond: TFLLR application (10 psi, 100 ms, 500 μ M). Adapted with permission from Di Castro et al. (2011) (A,B) and Woo et al. (2012) (C–E).
Similar articles
- Glia-neuron intercommunications and synaptic plasticity.
Vernadakis A. Vernadakis A. Prog Neurobiol. 1996 Jun;49(3):185-214. doi: 10.1016/s0301-0082(96)00012-3. Prog Neurobiol. 1996. PMID: 8878303 Review. - Astroglial calcium signaling displays short-term plasticity and adjusts synaptic efficacy.
Sibille J, Zapata J, Teillon J, Rouach N. Sibille J, et al. Front Cell Neurosci. 2015 May 27;9:189. doi: 10.3389/fncel.2015.00189. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26074766 Free PMC article. - Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse.
Sibille J, Pannasch U, Rouach N. Sibille J, et al. J Physiol. 2014 Jan 1;592(1):87-102. doi: 10.1113/jphysiol.2013.261735. Epub 2013 Sep 30. J Physiol. 2014. PMID: 24081156 Free PMC article. - Perisynaptic astroglial processes: dynamic processors of neuronal information.
Ghézali G, Dallérac G, Rouach N. Ghézali G, et al. Brain Struct Funct. 2016 Jun;221(5):2427-42. doi: 10.1007/s00429-015-1070-3. Epub 2015 May 31. Brain Struct Funct. 2016. PMID: 26026482 Review. - Monitoring Interneuron-Astrocyte Signaling and Its Consequences on Synaptic Transmission.
Mederos S, Perea G. Mederos S, et al. Methods Mol Biol. 2019;1938:117-129. doi: 10.1007/978-1-4939-9068-9_9. Methods Mol Biol. 2019. PMID: 30617977
Cited by
- Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology?
Decrock E, De Bock M, Wang N, Bultynck G, Giaume C, Naus CC, Green CR, Leybaert L. Decrock E, et al. Cell Mol Life Sci. 2015 Aug;72(15):2823-51. doi: 10.1007/s00018-015-1962-7. Epub 2015 Jun 29. Cell Mol Life Sci. 2015. PMID: 26118660 Free PMC article. Review. - Mechanisms of the negative potential associated with Leão's spreading depolarization: A history of brain electrogenesis.
Herreras O, Makarova J. Herreras O, et al. J Cereb Blood Flow Metab. 2020 Oct;40(10):1934-1952. doi: 10.1177/0271678X20935998. Epub 2020 Jun 24. J Cereb Blood Flow Metab. 2020. PMID: 32580670 Free PMC article. Review. - Connexin-Mediated Functional and Metabolic Coupling Between Astrocytes and Neurons.
Mayorquin LC, Rodriguez AV, Sutachan JJ, Albarracín SL. Mayorquin LC, et al. Front Mol Neurosci. 2018 Apr 11;11:118. doi: 10.3389/fnmol.2018.00118. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29695954 Free PMC article. - Image-Based Profiling of Synaptic Connectivity in Primary Neuronal Cell Culture.
Verstraelen P, Van Dyck M, Verschuuren M, Kashikar ND, Nuydens R, Timmermans JP, De Vos WH. Verstraelen P, et al. Front Neurosci. 2018 Jun 26;12:389. doi: 10.3389/fnins.2018.00389. eCollection 2018. Front Neurosci. 2018. PMID: 29997468 Free PMC article. Review. - The neuroglial potassium cycle during neurotransmission: role of Kir4.1 channels.
Sibille J, Dao Duc K, Holcman D, Rouach N. Sibille J, et al. PLoS Comput Biol. 2015 Mar 31;11(3):e1004137. doi: 10.1371/journal.pcbi.1004137. eCollection 2015 Mar. PLoS Comput Biol. 2015. PMID: 25826753 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources