Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment - PubMed (original) (raw)
Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment
Chang You et al. Int J Neuropsychopharmacol. 2014 Feb.
Abstract
Development of anxiety-like behaviours during ethanol withdrawal has been correlated with increased histone deacetylase (HDAC) activity and decreased brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton-associated protein (Arc) gene expression in the amygdala. Furthermore, HDAC-mediated histone modifications play a role in synaptic plasticity. In this study we used the HDAC inhibitor trichostatin A (TSA) to determine whether HDAC inhibition could prevent ethanol withdrawal-induced deficits in dendritic spine density (DSD), BDNF or Arc expression in the amygdala of rats. It was found that decreased BDNF and Arc expression in the central (CeA) and medial nucleus of amygdala (MeA), observed during withdrawal after chronic ethanol exposure, were normalized following acute TSA treatment. TSA treatment was also able to attenuate anxiety-like behaviours during ethanol withdrawal and correct the observed decrease in DSD in the CeA and MeA of ethanol-withdrawn rats. Taken together, these findings demonstrate that correcting the deficits in histone acetylation through TSA treatment also amends downstream synaptic plasticity-related deficits such as BDNF and Arc expression, and DSD in the CeA and MeA as well as attenuates anxiety-like behaviours in rats during withdrawal after chronic ethanol exposure.
Conflict of interest statement
Conflict of Interest
SCP reports that a US patent application on a related topic (serial number 60/848237 filed on September 29th, 2006) is currently pending. All other authors reported no biomedical financial interests or potential conflicts of interest.
Figures
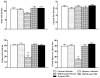
Figure 1
Measurement of anxiety-like behaviors using the elevated plus maze (EPM) shows increased anxiety levels in ethanol-withdrawn rats and attenuation of anxiety-like behaviors following TSA treatment. The anxiety-like behaviors are represented by open arm and closed arm activity. Values are the mean ± SEM of 6–8 rats in each group. * Significantly different (p < 0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test; percentage of open arm entries [_F_4, 27=21.25, p<.001] and the percentage of time spent in the open arms [_F_4, 27=17.141, p<.001]) from all other groups (Control+ Vehicle, Ethanol+ Vehicle, Withdrawal+ TSA, Control+ TSA injected rats).
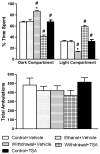
Figure 2
Measurement of anxiety-like behaviors in rats using light/dark box (LDB) shows increased anxiety levels in ethanol-withdrawn rats. TSA treatment prevents the withdrawal-induced anxiogenic effect. The data are represented as percentage of time spent in light and dark compartment of LDB. The general activities of the rats are measured by total ambulations during the exploration of LDB. Values are the mean ± SEM of 6–7 rats in each group. * Significantly different (p < 0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test; percentage of time spent in the light and dark compartments [_F_4, 26=43.71, p<.001]) from all other groups (Control+ Vehicle, Ethanol+ Vehicle, Withdrawal+ TSA, Control+ TSA injected rats). # Groups significantly different (p<0.001) from each other.
Figure 3
A, Representative photomicrographs of brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton-associated (Arc) protein gold-immunolabeling and Arc mRNA in situ RT-PCR in the central nucleus of amygdala (CeA) of various rat groups (Scale bar = 40 μm). B, Bar diagram showing the effects of chronic ethanol treatment and its withdrawal (with or without TSA treatment) on BDNF protein, Arc protein and mRNA levels in CeA, medial nucleus of amygdala (MeA) and basolateral amygdala (BLA) of rats. Values are mean ± SEM of 6 rats in each group. * Significantly different (p <0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test; [BDNF protein: CeA, _F_4,25=109.11 , p<.001; MeA, _F_4,25=100.51 , p<.001; Arc protein: CeA, _F_4,25=60.23, p<.001; MeA, _F_4,25=29.93, p<.001; Arc mRNA: CeA, _F_4,25=25.59, p<.001; MeA, _F_4,25=16.24, p<.001]) from all other groups (Control+ Vehicle, Ethanol+ Vehicle, Withdrawal+ TSA, Control+ TSA injected rats).
Figure 3
A, Representative photomicrographs of brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton-associated (Arc) protein gold-immunolabeling and Arc mRNA in situ RT-PCR in the central nucleus of amygdala (CeA) of various rat groups (Scale bar = 40 μm). B, Bar diagram showing the effects of chronic ethanol treatment and its withdrawal (with or without TSA treatment) on BDNF protein, Arc protein and mRNA levels in CeA, medial nucleus of amygdala (MeA) and basolateral amygdala (BLA) of rats. Values are mean ± SEM of 6 rats in each group. * Significantly different (p <0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test; [BDNF protein: CeA, _F_4,25=109.11 , p<.001; MeA, _F_4,25=100.51 , p<.001; Arc protein: CeA, _F_4,25=60.23, p<.001; MeA, _F_4,25=29.93, p<.001; Arc mRNA: CeA, _F_4,25=25.59, p<.001; MeA, _F_4,25=16.24, p<.001]) from all other groups (Control+ Vehicle, Ethanol+ Vehicle, Withdrawal+ TSA, Control+ TSA injected rats).
Figure 4
A, Representative photomicrographs of dendritic spines in the central nucleus of amygdala (CeA) and basolateral amygdala (BLA) of various rat groups as measured by Golgi-Cox staining (scale bar = 10 μm). B, Effect of chronic ethanol treatment and its withdrawal (with or without TSA treatment) on dendritic spine density in the amygdaloid brain regions of rats. Values are mean ± SEM of 6 rats in each group. *Significantly different [p <0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test; CeA, _F_4, 25=14.89, p<.001; Medial nucleus of amygdala (MeA), _F_4, 25=13.51, p<.001] from all other groups (Control+ Vehicle, Ethanol+ Vehicle, Withdrawal+ TSA, Control+ TSA injected rats).
Similar articles
- Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood.
Pandey SC, Sakharkar AJ, Tang L, Zhang H. Pandey SC, et al. Neurobiol Dis. 2015 Oct;82:607-619. doi: 10.1016/j.nbd.2015.03.019. Epub 2015 Mar 24. Neurobiol Dis. 2015. PMID: 25814047 Free PMC article. - Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism.
Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Pandey SC, et al. J Neurosci. 2008 Mar 5;28(10):2589-600. doi: 10.1523/JNEUROSCI.4752-07.2008. J Neurosci. 2008. PMID: 18322102 Free PMC article. - The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism.
Moonat S, Sakharkar AJ, Zhang H, Pandey SC. Moonat S, et al. Addict Biol. 2011 Apr;16(2):238-50. doi: 10.1111/j.1369-1600.2010.00275.x. Epub 2010 Dec 23. Addict Biol. 2011. PMID: 21182574 Free PMC article. - [Epigenetic mechanisms and alcohol use disorders: a potential therapeutic target].
Legastelois R, Jeanblanc J, Vilpoux C, Bourguet E, Naassila M. Legastelois R, et al. Biol Aujourdhui. 2017;211(1):83-91. doi: 10.1051/jbio/2017014. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682229 Review. French. - Dendritic spines of the medial amygdala: plasticity, density, shape, and subcellular modulation by sex steroids.
Rasia-Filho AA, Dalpian F, Menezes IC, Brusco J, Moreira JE, Cohen RS. Rasia-Filho AA, et al. Histol Histopathol. 2012 Aug;27(8):985-1011. doi: 10.14670/HH-27.985. Histol Histopathol. 2012. PMID: 22763872 Review.
Cited by
- Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood.
Pandey SC, Sakharkar AJ, Tang L, Zhang H. Pandey SC, et al. Neurobiol Dis. 2015 Oct;82:607-619. doi: 10.1016/j.nbd.2015.03.019. Epub 2015 Mar 24. Neurobiol Dis. 2015. PMID: 25814047 Free PMC article. - Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons.
You C, Vandegrift B, Brodie MS. You C, et al. Psychopharmacology (Berl). 2018 Jun;235(6):1711-1726. doi: 10.1007/s00213-018-4875-y. Epub 2018 Mar 16. Psychopharmacology (Berl). 2018. PMID: 29549390 Free PMC article. Review. - Epigenetic mechanisms of alcoholism and stress-related disorders.
Palmisano M, Pandey SC. Palmisano M, et al. Alcohol. 2017 May;60:7-18. doi: 10.1016/j.alcohol.2017.01.001. Epub 2017 Mar 3. Alcohol. 2017. PMID: 28477725 Free PMC article. Review. - Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats.
Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson DR, Pandey SC. Sakharkar AJ, et al. Int J Neuropsychopharmacol. 2014 Dec;17(12):2057-67. doi: 10.1017/S1461145714001047. Epub 2014 Jun 26. Int J Neuropsychopharmacol. 2014. PMID: 24968059 Free PMC article. - Alcohol Withdrawal and Cerebellar Mitochondria.
Jung ME. Jung ME. Cerebellum. 2015 Aug;14(4):421-37. doi: 10.1007/s12311-014-0598-8. Cerebellum. 2015. PMID: 25195804 Review.
References
- Camelo S, Iglesias AH, Hwang D, Due B, Ryu H, Smith K, Gray SG, Imitola J, Duran G, Assaf B, Langley B, Khoury SJ, Stephanopoulos G, De Girolami U, Ratan RR, Ferrante RJ, Dangond F. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164(1–2):10–21. - PubMed
- Colzato LS, Van der Does AJ, Kouwenhoven C, Elzinga BM, Hommel B. BDNF Val66Met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology. 2011;36(10):1562–1569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R29 AA010005/AA/NIAAA NIH HHS/United States
- U01 AA019971/AA/NIAAA NIH HHS/United States
- AA-019971/AA/NIAAA NIH HHS/United States
- AA-016690/AA/NIAAA NIH HHS/United States
- R01 AA010005/AA/NIAAA NIH HHS/United States
- R01 AA016690/AA/NIAAA NIH HHS/United States
- R01 AA013341/AA/NIAAA NIH HHS/United States
- AA-013341/AA/NIAAA NIH HHS/United States
- AA-010005/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials