Antiobesity pharmacotherapy: new drugs and emerging targets - PubMed (original) (raw)
Review
Antiobesity pharmacotherapy: new drugs and emerging targets
G W Kim et al. Clin Pharmacol Ther. 2014 Jan.
Abstract
Obesity is a growing pandemic, and related health and economic costs are staggering. Pharmacotherapy, partnered with lifestyle modifications, forms the core of current strategies to reduce the burden of this disease and its sequelae. However, therapies targeting weight loss have a significant history of safety risks, including cardiovascular and psychiatric events. Here, evolving strategies for developing antiobesity therapies, including targets, mechanisms, and developmental status, are highlighted. Progress in this field is underscored by Belviq (lorcaserin) and Qsymia (phentermine/topiramate), the first agents in more than 10 years to achieve regulatory approval for chronic weight management in obese patients. On the horizon, novel insights into metabolism and energy homeostasis reveal guanosine 3',5'-cyclic monophosphate (cGMP) signaling circuits as emerging targets for antiobesity pharmacotherapy. These innovations in molecular discovery may elegantly align with practical off-the-shelf approaches, leveraging existing approved drugs that modulate cGMP levels for the management of obesity.
Figures
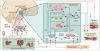
Figure 1. Central regulation of appetite and energy homoeostasis
The hypothalamus is the major site of integration of anorexigenic and orexigenic signaling. Peripheral satiety hormones, such as ghrelin from the stomach and leptin from adipose tissue, primarily bind and activate their cognate receptors directly in the hypothalamus, particularly in the arcuate nucleus, or in the dorsal vagal complex in the medulla, which communicates with the hypothalamus. Among the neurons in the arcuate nucleus there exist two populations of neurons: those expressing the orexigenic neuropeptide Y (NPY) or agouti-related peptide (AgRP); and those expressing the anorexigenic pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART). Several satiety hormones induce their anorectic effects by either inhibiting the activity of NPY/AgRP neurons or activating POMC/CART neurons. These neurons in the arcuate nucleus project to second-order neurons in other hypothalamic nuclei, including the paraventricular nucleus (PVN), dorsomedial nucleus (DMN), ventromedial nucleus (VMN), and lateral hypothalamic area (LHA). These second-order hypothalamic neurons express anorexigenic neuropeptides (corticotropin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH), brain-derived neurotrophic factor (BDNF)) and orexigenic neuropeptides (orexin (ORX), melanin-concentrating hormone (MCH)), which modulate appetite and energy homeostasis. Furthermore, the regulation of energy balance involves an integration of signaling from the hypothalamus, brainstem, and reward pathways of the mesolimbic system. Symbols: blue receptor = activating; red receptor = inhibiting; blue arrow = appetite-stimulating; red arrow = appetite-suppressing.
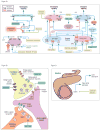
Figure 2. Molecular targets for anti-obesity pharmacotherapeutics
(2a) Phentermine stimulates the release of dopamine (DA) into the synapse from the presynaptic dopaminergic neurons from the ventral tegmental area (VTA). This released dopamine binds and activates dopamine receptors, including D1 and D2, on postsynaptic neurons in the nucleus accumbens. Dopamine is taken back up into the presynaptic neuron by the dopamine transporter (DAT), which is bound and inhibited by bupropion. Pramlintide and davalintide are amylin analogs that activate amylin receptors (CTR) in the dorsal vagal complex (DVC). Exenatide, liraglutide, and NN9924 are GLP-1 analogs that activate GLP-1 receptors (GLP-1R) in the hypothalamus and DVC. S-2367 binds and inhibits Y5 receptors in the hypothalamic paraventricular nucleus (PVN) to suppress neuropeptide Y (NPY) signaling. RM493 is a melanocortin 4 receptor (MC4R) agonist that binds and activates MC4R in the PVN and lateral hypothalamic area (LHA). Topiramate induces gamma-aminobutyric acid (GABA) receptor-mediated inhibitory currents. TKS1225 and OXY-RPEG are oxyntomodulin (OXM) analogs that bind and activate GLP-1R in the arcuate nucleus and DVC. Lorcaserin selectively binds and activates 5-HT2c serotonin receptors in the arcuate nucleus. Symbols: blue receptor = activating; red receptor = inhibiting. (2b) Topiramate's exact mechanism of action is unknown, but may involve its ability to block voltage-gated sodium channels in the presynaptic excitatory neuron; antagonize AMPA/NMDA glutamate receptors on postsynaptic neurons; and enhance the activity of inhibitory GABA neurons. (2c) Orlistat binds and inhibits gastric and pancreatic lipases in the intestine. These lipases hydrolyze dietary triglycerides into free fatty acids that can be absorbed by intestinal epithelial cells via fatty acid transporters. Thus, orlistat suppresses systemic lipid absorption.
Figure 3. Cyclic AMP- and GMP-mediated signaling regulates metabolism and energy homeostasis
Increased cGMP levels, via activation of particulate or soluble guanylyl cyclases (pGC or sGC), activates peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), a master regulator of mitochondrial biogenesis and function. Cyclic GMP signaling also regulates the metabolic sensors SIRT1 and AMP-activated protein kinase (AMPK), which directly activate PGC-1α by deacetylation and phosphorylation, respectively. Resveratrol stimulates mitochondrial function by activating SIRT1 and stimulating cGMP production. Phosphodiesterases (PDE), which hydrolyze cAMP and cGMP and thereby terminate their signal, are the target for sildenafil, a PDE inhibitor specific for PDE5. Also, cAMP is required for glucose-induced insulin release by pancreatic β-cells. Furthermore, postprandial release of the intestinal hormone uroguanylin results in the endocrine activation of hypothalamic guanylyl cyclase C (GUCY2C) to produce cGMP, which stimulates satiety and suppresses appetite. Also, in adipose tissue, cGMP signaling promotes lipolysis, and induces adipogenic and thermogenic programs during brown fat cell differentiation through PKG-mediated inhibition of RhoA and Rho-associated kinase (ROCK), and thereby activation of phosphoinositide 3-kinase-AKT signaling.
Similar articles
- New antiobesity agents: lorcaserin (Belviq) and phentermine/topiramate ER (Qsymia).
Shyh G, Cheng-Lai A. Shyh G, et al. Cardiol Rev. 2014 Jan-Feb;22(1):43-50. doi: 10.1097/CRD.0000000000000001. Cardiol Rev. 2014. PMID: 24304809 Review. - Lorcaserin and phentermine/topiramate: two leaps forward in weight loss pharmacotherapy.
Hill LG. Hill LG. Ann Pharmacother. 2013 Dec;47(12):1740. doi: 10.1177/1060028013513557. Ann Pharmacother. 2013. PMID: 24396110 No abstract available. - Response to "lorcaserin and phentermine/topiramate: two leaps forward in weight loss pharmacotherapy".
Fleming JW, McClendon KS, Riche DM. Fleming JW, et al. Ann Pharmacother. 2013 Dec;47(12):1741. doi: 10.1177/1060028013513884. Epub 2013 Dec 5. Ann Pharmacother. 2013. PMID: 24311729 No abstract available. - New obesity agents: lorcaserin and phentermine/topiramate.
Fleming JW, McClendon KS, Riche DM. Fleming JW, et al. Ann Pharmacother. 2013 Jul-Aug;47(7-8):1007-16. doi: 10.1345/aph.1R779. Epub 2013 Jun 25. Ann Pharmacother. 2013. PMID: 23800750 Review. - Phentermine/topiramate for the treatment of obesity.
Smith SM, Meyer M, Trinkley KE. Smith SM, et al. Ann Pharmacother. 2013 Mar;47(3):340-9. doi: 10.1345/aph.1R501. Ann Pharmacother. 2013. PMID: 23482732 Review.
Cited by
- Reverse Engineering Drugs: Lorcaserin as an Example.
Schwasinger-Schmidt T, Preskorn SH. Schwasinger-Schmidt T, et al. Adv Neurobiol. 2023;30:195-206. doi: 10.1007/978-3-031-21054-9_8. Adv Neurobiol. 2023. PMID: 36928851 - Treatment of Acquired Hypothalamic Obesity: Now and the Future.
Dimitri P. Dimitri P. Front Endocrinol (Lausanne). 2022 Apr 6;13:846880. doi: 10.3389/fendo.2022.846880. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35464063 Free PMC article. Review. - The effects of ginger and its constituents in the prevention of metabolic syndrome: A review.
Salaramoli S, Mehri S, Yarmohammadi F, Hashemy SI, Hosseinzadeh H. Salaramoli S, et al. Iran J Basic Med Sci. 2022 Jun;25(6):664-674. doi: 10.22038/IJBMS.2022.59627.13231. Iran J Basic Med Sci. 2022. PMID: 35949312 Free PMC article. Review. - Pharmacological Treatments and Natural Biocompounds in Weight Management.
Gasmi A, Mujawdiya PK, Nehaoua A, Shanaida M, Semenova Y, Piscopo S, Menzel A, Voloshyn V, Voloshyn O, Shanaida V, Bjørklund G. Gasmi A, et al. Pharmaceuticals (Basel). 2023 Jan 30;16(2):212. doi: 10.3390/ph16020212. Pharmaceuticals (Basel). 2023. PMID: 37139804 Free PMC article. Review. - An overview on recent in vivo biological application of cerium oxide nanoparticles.
Stephen Inbaraj B, Chen BH. Stephen Inbaraj B, et al. Asian J Pharm Sci. 2020 Sep;15(5):558-575. doi: 10.1016/j.ajps.2019.10.005. Epub 2019 Nov 27. Asian J Pharm Sci. 2020. PMID: 33193860 Free PMC article. Review.
References
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nature reviews Endocrinology. 2012;9:13–27. - PubMed
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–25. - PubMed
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. - PubMed
- AMA Adopts New Policies on Second Day of Voting at Annual Meeting. 2013 http://www.ama-assn.org/ama/pub/news/news/2013/2013-06-18-new-ama-polici....
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nature reviews Endocrinology. 2013;9:13–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA170533/CA/NCI NIH HHS/United States
- F30 CA180500/CA/NCI NIH HHS/United States
- T32 GM08562/GM/NIGMS NIH HHS/United States
- 1 F30 CA180500/CA/NCI NIH HHS/United States
- P30 CA56036/CA/NCI NIH HHS/United States
- T32 GM008562/GM/NIGMS NIH HHS/United States
- P30 CA056036/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical