Toggling a conformational switch in Wnt/β-catenin signaling: regulation of Axin phosphorylation. The phosphorylation state of Axin controls its scaffold function in two Wnt pathway protein complexes - PubMed (original) (raw)
Review
Toggling a conformational switch in Wnt/β-catenin signaling: regulation of Axin phosphorylation. The phosphorylation state of Axin controls its scaffold function in two Wnt pathway protein complexes
Ofelia Tacchelly-Benites et al. Bioessays. 2013 Dec.
Abstract
The precise orchestration of two opposing protein complexes - one in the cytoplasm (β-catenin destruction complex) and the other at the plasma membrane (LRP6 signaling complex) - is critical for controlling levels of the transcriptional co-factor β-catenin, and subsequent activation of the Wnt/β-catenin signal transduction pathway. The Wnt pathway component Axin acts as an essential scaffold for the assembly of both complexes. How the β-catenin destruction and LRP6 signaling complexes are modulated following Wnt stimulation remains controversial. A recent study in Science by He and coworkers reveals an underlying logic for Wnt pathway control in which Axin phosphorylation toggles a switch between the active and inactive states. This mini-review focuses on this and two other recent studies that provide insight into the initial signaling events triggered by Wnt exposure. We emphasize regulation of the β-catenin destruction and LRP6 signaling complexes and propose a framework for future work in this area.
Keywords: Axin; LRP6 signaling complex; PP1; Wnt signal transduction; β-catenin destruction complex.
© 2013 WILEY Periodicals, Inc.
Figures
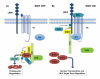
Figure 1. Wnt/β-catenin Signaling
A) The β-catenin destruction complex. In the absence of Wnt stimulation, steady-state levels of β-catenin are maintained via its constitutive synthesis and proteolysis. The Axin scaffold facilitates the association of β-catenin, GSK3, CK1α , and APC. Phosphorylation of β-catenin by CK1α and GSK3 promotes its recognition by the E3 ubiquitin ligase β-TrCP, targeting β-catenin for proteasomal degradation. B) The LRP6 signaling complex. Wnt exposure induces formation of a receptor complex between Fz, LRP6, and Wnt, and recruitment of Dvl to Fz. Formation of this complex triggers phosphorylation of LRP6 by GSK3 and CK1, and subsequent recruitment of Axin and GSK3 to phospho-LRP6, which results in increased LRP6 phosphorylation. Formation of the LRP6 signaling complex results in inactivation of the destruction complex, leading to β-catenin stabilization, nuclear translocation, and a Wnt-specific transcriptional program.
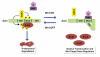
Figure 2. The Axin conformation controls its scaffold function
Left. In the absence of Wnt, Axin is phosphorylated by GSK3 at the S497/500 sites, located within the central β-catenin binding domain (BCD). Axin phosphorylation results in an “open” conformation that promotes its interaction with β-catenin, targeting β-catenin for ubiquitin-dependent proteolysis. The open conformation also primes Axin for interaction with LRP6. GID: GSK3 interaction domain; DIX: Dishevelled interaction domain. Right. In the presence of Wnt, Axin is dephosphorylated by PP1 at the S497/500 sites. Following its dephosphorylation, the BCD of Axin associates with its carboxy-terminal DIX domain, resulting in a “closed” conformation. This intramolecular association prevents the interaction of Axin with both LRP6 and β-catenin, thereby inactivating Axin.
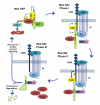
Figure 3. The Axin phosphorylation/dephosphorylation cycle
Top left, in the absence of Wnt. Axin is phosphorylated by GSK3, maintained in an open conformation, engaged in the destruction complex, and primed for interaction with LRP6. Top right, Phase I following Wnt exposure. LRP6 undergoes phosphorylation. Phospho-Axin is recruited from the destruction complex to the LRP6 signaling complex through its interaction with phospho-LRP6. Bottom right, Phase II following Wnt exposure. The LRP6 signaling complex inhibits GSK3, thereby tipping the balance towards Axin dephosphorylation by PP1. Bottom left, Phase III following Wnt exposure. Dephosphorylation of Axin by PP1 results in a closed conformation, thereby disengaging Axin from LRP6 and β-catenin. Phospho-LRP6 becomes free for additional rounds of engagement with phospho-Axin, leading to further dephosphorylation and inactivation of Axin. Dephosphorylated Axin is subsequently targeted for proteasomal degradation or recycled for destruction complex assembly.
Similar articles
- Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies.
Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, MacDonald BT, Zhang X, Garcia Abreu J, Peng L, He X. Kim SE, et al. Science. 2013 May 17;340(6134):867-70. doi: 10.1126/science.1232389. Epub 2013 Apr 11. Science. 2013. PMID: 23579495 Free PMC article. - Destruction complex dynamics: Wnt/β-catenin signaling alters Axin-GSK3β interactions in vivo.
Lybrand DB, Naiman M, Laumann JM, Boardman M, Petshow S, Hansen K, Scott G, Wehrli M. Lybrand DB, et al. Development. 2019 Jul 2;146(13):dev164145. doi: 10.1242/dev.164145. Development. 2019. PMID: 31189665 Free PMC article. - LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin.
Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. Cselenyi CS, et al. Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8032-7. doi: 10.1073/pnas.0803025105. Epub 2008 May 28. Proc Natl Acad Sci U S A. 2008. PMID: 18509060 Free PMC article. - New insights into the regulation of Axin function in canonical Wnt signaling pathway.
Song X, Wang S, Li L. Song X, et al. Protein Cell. 2014 Mar;5(3):186-93. doi: 10.1007/s13238-014-0019-2. Epub 2014 Jan 29. Protein Cell. 2014. PMID: 24474204 Free PMC article. Review. - Wnt/Beta-Catenin Signaling Regulation and a Role for Biomolecular Condensates.
Schaefer KN, Peifer M. Schaefer KN, et al. Dev Cell. 2019 Feb 25;48(4):429-444. doi: 10.1016/j.devcel.2019.01.025. Dev Cell. 2019. PMID: 30782412 Free PMC article. Review.
Cited by
- An early global role for Axin is required for correct patterning of the anterior-posterior axis in the sea urchin embryo.
Sun H, Peng CJ, Wang L, Feng H, Wikramanayake AH. Sun H, et al. Development. 2021 Mar 31;148(7):dev191197. doi: 10.1242/dev.191197. Development. 2021. PMID: 33688076 Free PMC article. - Reversal of hyperactive Wnt signaling-dependent adipocyte defects by peptide boronic acids.
Zhang T, Hsu FN, Xie XJ, Li X, Liu M, Gao X, Pei X, Liao Y, Du W, Ji JY. Zhang T, et al. Proc Natl Acad Sci U S A. 2017 Sep 5;114(36):E7469-E7478. doi: 10.1073/pnas.1621048114. Epub 2017 Aug 21. Proc Natl Acad Sci U S A. 2017. PMID: 28827348 Free PMC article. - Tankyrases: structure, function and therapeutic implications in cancer.
Haikarainen T, Krauss S, Lehtio L. Haikarainen T, et al. Curr Pharm Des. 2014;20(41):6472-88. doi: 10.2174/1381612820666140630101525. Curr Pharm Des. 2014. PMID: 24975604 Free PMC article. Review. - Identification of protein phosphatase 4 catalytic subunit as a Wnt promoting factor in pan-cancer and Xenopus early embryogenesis.
Wang Y, Han W, Yun S, Han J. Wang Y, et al. Sci Rep. 2023 Jun 23;13(1):10240. doi: 10.1038/s41598-023-35719-y. Sci Rep. 2023. PMID: 37353511 Free PMC article. - Autosomal Recessive Primary Microcephaly: Not Just a Small Brain.
Zaqout S, Kaindl AM. Zaqout S, et al. Front Cell Dev Biol. 2022 Jan 17;9:784700. doi: 10.3389/fcell.2021.784700. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35111754 Free PMC article. Review.
References
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205. - PubMed
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–7. - PubMed
- Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25:254–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM103926/GM/NIGMS NIH HHS/United States
- R01 CA105038/CA/NCI NIH HHS/United States
- R01GM081635/GM/NIGMS NIH HHS/United States
- R01 GM081635/GM/NIGMS NIH HHS/United States
- R01CA105038/CA/NCI NIH HHS/United States
- R01 GM122222/GM/NIGMS NIH HHS/United States
- R01GM103926/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources