Structure of an RNA silencing complex of the CRISPR-Cas immune system - PubMed (original) (raw)
Comparative Study
Structure of an RNA silencing complex of the CRISPR-Cas immune system
Michael Spilman et al. Mol Cell. 2013.
Abstract
Bacterial and archaeal clustered regularly interspaced short palindromic repeat (CRISPR) loci capture virus and plasmid sequences and use them to recognize and eliminate these invaders. CRISPR RNAs (crRNAs) containing the acquired sequences are incorporated into effector complexes that destroy matching invader nucleic acids. The multicomponent Cmr effector complex cleaves RNA targets complementary to the crRNAs. Here, we report cryoelectron microscopy reconstruction of a functional Cmr complex bound with a target RNA at ~12 Å. Pairs of the Cmr4 and Cmr5 proteins form a helical core that is asymmetrically capped on each end by distinct pairs of the four remaining subunits: Cmr2 and Cmr3 at the conserved 5' crRNA tag sequence and Cmr1 and Cmr6 near the 3' end of the crRNA. The shape and organization of the RNA-targeting Cmr complex is strikingly similar to the DNA-targeting Cascade complex. Our results reveal a remarkably conserved architecture among very distantly related CRISPR-Cas complexes.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Fig. 1
Overview of the P. furiosus CRISPR/Cas locus and the Cmr complex structure determined by cryo electron microscopy. (A) The genes encoding the Cmr complex protein subunits are color-coded to match those used for structure images in subsequent figures. The CRISPR repeats are shown in black and spacers in various colors. (B) The mature 39-nt and 45-nt crRNAs contain a 5′ repeat-derived 8-nt sequence (5′-tag) and a 31-nt or 37-nt spacer-derived sequence (guide), respectively. The sequence of the target RNA used in RNA cleavage assays and assembly with the Cmr complex is shown in blue. (C) Color-coded EM density of the Cmr complex bound with the 45-nt crRNA and the target RNA is shown in two orientations. Cmr1 (red), Cmr2 (light blue), Cmr3 (orange), Cmr4 (three different shades of green), Cmr5 (three different shades of yellow), and Cmr6 (magenta). (D) Fitting of the crystal structure of Cmr2-Cmr3 complex to the ‘foot’ of the Cmr complex in two orthogonal orientations. (E) Helical reconstruction of the Cmr4-Cmr5 filament structure. Circled regions indicate matched densities in both the Cmr complex and the Cmr4-Cmr5 filament. See also Figures S1, S2 and S3.
Fig. 2
Assembly process of the Cmr complex. (A) Left, a ribbon model of the assembled protein subunits of an intact target-bound Cmr complex. Right, capping at the foot of the Cmr complex by interaction of the homologous domain of Cmr2 with Cmr5 and of Cmr3 with Cmr4. Structural similarity between Cmr5 ((Park et al., 2013), PDB ID 4GKF) and the D4 domain of Cmr2 is shown (r.m.s.d = 3.3 Å for 62 aligned Cα atoms). A homology model of Cmr4 constructed from Cas6 (sequence similarity 30%, (Carte et al., 2008), PDB ID 3PKM) is also homologous to that of Cmr3 (r.m.s.d = 3.4 Å for 136 aligned Cα atoms). (B) A micrograph of the Cmr4-Cmr5 filament and the class averages of Cmr4-Cmr5 filaments. (C) Model for assembly method of the Cmr complex. By acting as a non-extendable Cmr4-Cmr5 dimer, Cmr6 and Cmr2-Cmr3 complex cap the growth of the Cmr4-Cmr5 filament at the top and foot of the Cmr complex, respectively. See also Figures S2E and S4.
Fig. 3
Positions of the crRNA and target RNA within the complex. (A) RNA Substrates used for crosslinking assays. The 45-nt crRNAs (1-3, 5-8) or tag RNAs (4) were generated by in vitro transcription and subsequent Cas6 digestion. Regions marked in yellow contain radiolabeled nucleotides. Labeled (i.e. crosslinked) (B) and Coomassie-stained (i.e. total) (C) proteins are shown. The RNA from (A) used in each reaction is indicated beneath the lanes, and correlates with (A). T1 and A denote RNases used in the experiment and visible in the Coomassie-stained gel (C). (D) Difference density to identify bound target RNA. The difference density was obtained by subtracting the negatively stained density of the Cmr complex in the absence of target RNA from that in the presence of target RNA. Left, the common density +/- target RNA is shown in yellow and the difference density in blue (10σ). Right, the same view as that on the left but clipped to expose the difference density in the center of the complex. (E) Schematic structure of the holo Cmr complex showing the deduced path of the crRNA and target RNA. See also Table S1.
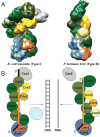
Fig. 4
Conserved organization of an RNA- and DNA-targeting CRISPR-Cas effector complex. The Cse and Cmr CRISPR-Cas systems include distantly related members of several broad categories of Cas proteins: large subunit (blue), small subunit (shades of yellow), Cas5 superfamily (orange), Cas7 superfamily (shades of green) and Cas6 superfamily proteins (gray). Our findings reveal a conserved functional organization of these classes of Cas proteins within the effector complexes. (A). Comparison of cryoEM structures of E. coli Cascade (left) and P. furiosus Cmr complex (right). Segmented densities assigned to the subunits are color-coded according to their broad classification. (B). Cartoon representations of the functional organization of the complexes. The Cas5 superfamily RAMP proteins – Cmr3 and Cas5e – are found near the 5′ tag of the crRNAs in both complexes. Large subunit proteins – Cmr2 (member of the Cas10 superfamily) and Cse1 (member of the Cas8 superfamily) – are also found near the 5′ end of the crRNAs. Cas7 superfamily RAMP proteins – Cmr4, Cmr6 and Cmr1 in the Cmr complex and Cse4 in the Cse complex – and the small subunit of proteins – Cmr5 and Cse2 – form the backbones of the helical structure that extends along the guide region of the crRNAs. In Cascade, the Cas6 superfamily RAMP protein – Cse3 – remains associated with the CRISPR repeat sequence retained at the 3′ end of the crRNA. The Cse complex binds DNA targets that are then cleaved by Cas3. The Cmr complex cleaves complementary RNAs.
Comment in
- Same same but different: new structural insight into CRISPR-Cas complexes.
Heidrich N, Vogel J. Heidrich N, et al. Mol Cell. 2013 Oct 10;52(1):4-7. doi: 10.1016/j.molcel.2013.09.023. Mol Cell. 2013. PMID: 24119398
Similar articles
- Target RNA capture and cleavage by the Cmr type III-B CRISPR-Cas effector complex.
Hale CR, Cocozaki A, Li H, Terns RM, Terns MP. Hale CR, et al. Genes Dev. 2014 Nov 1;28(21):2432-43. doi: 10.1101/gad.250712.114. Genes Dev. 2014. PMID: 25367038 Free PMC article. - Crystal structure of the CRISPR-Cas RNA silencing Cmr complex bound to a target analog.
Osawa T, Inanaga H, Sato C, Numata T. Osawa T, et al. Mol Cell. 2015 May 7;58(3):418-30. doi: 10.1016/j.molcel.2015.03.018. Epub 2015 Apr 23. Mol Cell. 2015. PMID: 25921071 - Crystal structure of the Cmr2-Cmr3 subcomplex in the CRISPR-Cas RNA silencing effector complex.
Osawa T, Inanaga H, Numata T. Osawa T, et al. J Mol Biol. 2013 Oct 23;425(20):3811-23. doi: 10.1016/j.jmb.2013.03.042. Epub 2013 Apr 10. J Mol Biol. 2013. PMID: 23583914 - The RNA- and DNA-targeting CRISPR-Cas immune systems of Pyrococcus furiosus.
Terns RM, Terns MP. Terns RM, et al. Biochem Soc Trans. 2013 Dec;41(6):1416-21. doi: 10.1042/BST20130056. Biochem Soc Trans. 2013. PMID: 24256230 Free PMC article. Review. - Approaches to study CRISPR RNA biogenesis and the key players involved.
Behler J, Hess WR. Behler J, et al. Methods. 2020 Feb 1;172:12-26. doi: 10.1016/j.ymeth.2019.07.015. Epub 2019 Jul 17. Methods. 2020. PMID: 31325492 Review.
Cited by
- CRISPR-based technologies: prokaryotic defense weapons repurposed.
Terns RM, Terns MP. Terns RM, et al. Trends Genet. 2014 Mar;30(3):111-8. doi: 10.1016/j.tig.2014.01.003. Epub 2014 Feb 18. Trends Genet. 2014. PMID: 24555991 Free PMC article. Review. - Origins and evolution of CRISPR-Cas systems.
Koonin EV, Makarova KS. Koonin EV, et al. Philos Trans R Soc Lond B Biol Sci. 2019 May 13;374(1772):20180087. doi: 10.1098/rstb.2018.0087. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30905284 Free PMC article. Review. - Systematic prediction of genes functionally linked to CRISPR-Cas systems by gene neighborhood analysis.
Shmakov SA, Makarova KS, Wolf YI, Severinov KV, Koonin EV. Shmakov SA, et al. Proc Natl Acad Sci U S A. 2018 Jun 5;115(23):E5307-E5316. doi: 10.1073/pnas.1803440115. Epub 2018 May 21. Proc Natl Acad Sci U S A. 2018. PMID: 29784811 Free PMC article. - Cas6 specificity and CRISPR RNA loading in a complex CRISPR-Cas system.
Sokolowski RD, Graham S, White MF. Sokolowski RD, et al. Nucleic Acids Res. 2014 Jun;42(10):6532-41. doi: 10.1093/nar/gku308. Epub 2014 Apr 20. Nucleic Acids Res. 2014. PMID: 24753403 Free PMC article. - RNA targeting by the type III-A CRISPR-Cas Csm complex of Thermus thermophilus.
Staals RH, Zhu Y, Taylor DW, Kornfeld JE, Sharma K, Barendregt A, Koehorst JJ, Vlot M, Neupane N, Varossieau K, Sakamoto K, Suzuki T, Dohmae N, Yokoyama S, Schaap PJ, Urlaub H, Heck AJ, Nogales E, Doudna JA, Shinkai A, van der Oost J. Staals RH, et al. Mol Cell. 2014 Nov 20;56(4):518-30. doi: 10.1016/j.molcel.2014.10.005. Epub 2014 Nov 6. Mol Cell. 2014. PMID: 25457165 Free PMC article.
References
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. - PubMed
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. - PubMed
- Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–2561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM054682/GM/NIGMS NIH HHS/United States
- R01 GM099604/GM/NIGMS NIH HHS/United States
- R01 GM54682/GM/NIGMS NIH HHS/United States
- R01 GM99604/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources