Can nanomedicines kill cancer stem cells? - PubMed (original) (raw)
Review
Can nanomedicines kill cancer stem cells?
Yi Zhao et al. Adv Drug Deliv Rev. 2013 Nov.
Abstract
Most tumors are heterogeneous and many cancers contain small population of highly tumorigenic and intrinsically drug resistant cancer stem cells (CSCs). Like normal stem cell, CSCs have the ability to self-renew and differentiate to other tumor cell types. They are believed to be a source for drug resistance, tumor recurrence and metastasis. CSCs often overexpress drug efflux transporters, spend most of their time in non-dividing G0 cell cycle state, and therefore, can escape the conventional chemotherapies. Thus, targeting CSCs is essential for developing novel therapies to prevent cancer relapse and emerging of drug resistance. Nanocarrier-based therapeutic agents (nanomedicines) have been used to achieve longer circulation times, better stability and bioavailability over current therapeutics. Recently, some groups have successfully applied nanomedicines to target CSCs to eliminate the tumor and prevent its recurrence. These approaches include 1) delivery of therapeutic agents (small molecules, siRNA, antibodies) that affect embryonic signaling pathways implicated in self-renewal and differentiation in CSCs, 2) inhibiting drug efflux transporters in an attempt to sensitize CSCs to therapy, 3) targeting metabolism in CSCs through nanoformulated chemicals and field-responsive magnetic nanoparticles and carbon nanotubes, and 4) disruption of multiple pathways in drug resistant cells using combination of chemotherapeutic drugs with amphiphilic Pluronic block copolymers. Despite clear progress of these studies the challenges of targeting CSCs by nanomedicines still exist and leave plenty of room for improvement and development. This review summarizes biological processes that are related to CSCs, overviews the current state of anti-CSCs therapies, and discusses state-of-the-art nanomedicine approaches developed to kill CSCs.
Keywords: Cancer; Cancer stem cells; Drug delivery; Nanomedicine; Polymer therapeutics.
© 2013.
Figures
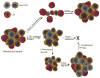
Fig. 1. Treatment of hierarchical tumors
(1) Treatment with most commonly used chemotherapeutic drugs (classic chemotherapy) often results in reduction in tumor volume, but drug-resistant CSCs can survive, repopulate the tumor and spread to distal sites (“tumor regrowth”). (2) Treatment of hierarchical cancers with therapeutic agents that target CSCs can kill CSCs and result in tumor remission. (3) According to the dynamic CSCs model differentiated cancer cells can reverse their phenotype and acquire CSCs properties (“phenotype reversal”). Therefore even if CSCs are selectively eliminated (2) the remaining cancer cells that reverse CSC phenotype will result in the tumor regrowth. (4) Combination treatment with the CSCs targeting drugs and conventional chemotherapies that reduce both CSCs and stromal cells may be an ideal regimen to achieve durable response.
Fig. 2. Schematic showing the main nanoparticles and microparticles investigated in the drug delivery applications
Microphotograph insert presents images of PRINT® (“Particle Replication In Non-Wetting Templates”) microparticles from the laboratory of Prof. DeSimone at the University of North Carolina at Chapel Hill provided by his graduate students T. Shen and C. Fromen.
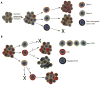
Fig. 3. Clonal evolution model (A) vs. CSC model (B)
(A) Clonal evolution model is a non-hierarchical model where stochastic genetic and/or epigenetic changes confer growth advantage as well as heritable phenotypic and functional differences. Clones can be different in their tumorigenicity and will produce cells with similar tumorigenic potential (e.g., Clone 1 and Clone 2). However some cells may lose their tumorigenic capacity due to non-favorable genetic/epigenetic changes or microenvironment (non-clonogenic cancer cells). (B) CSCs model is a hierarchical model: (1) non tumorigenic cancer cell cannot generate new tumor; (2) CSCs have the ability to generate a tumor, based on its self-renewal and tumorigenic properties; (3) clonal evolution in CSCs due to genetic and/or epigenetic changes in CSCs leads to expansion of CSCs pool, higher heterogeneity and possibly development of drug resistance; (4) recent studies demonstrated that CSCs phenotype is dynamic and non-stem cells may acquire CSCs properties (phenotype reversal), which eventually results in tumor recurrence.
Fig. 4. Models of MDR development
(A) Conventional model: rare cells in the tumor have accumulated significant genetic and/or epigenetic alterations that confer MDR. These cells will survive the chemotherapy and proliferate in MDR tumor. (B) CSCs model: tumor contains small population of CSCs and their differentiated progenies. The chemotherapy eliminates the differentiated cells, while the MDR CSCs survive and produce drug resistant heterogeneous tumor, consisting of CSCs and various differentiated (drug resistant) cells.
Fig. 5. Possible therapeutic strategies that can eliminate CSCs
(1) Targeting cell surface proteins (for example, CD133, CD44, et al.) to develop site-specific therapeutics against CSCs. (2) Inhibiting the drug efflux transporters to sensitize drug resistant CSCs to chemotherapeutic agents. (3) Altering CSCs metabolism by “reprogramming” it to “normal” status to impair growth of CSCs, induce differentiation of CSCs and, finally, sensitize CSCs to the therapy. (4) Inhibiting cell signaling pathways that are critical for the CSCs survival and proliferation to develop anti-CSCs therapeutic agents. (5) Affecting the vascular niche of the CSCs to impair the specialized microenvironment housing the CSCs.
Fig. 6
(A) Structure of Pluronic block copolymers along with empty and drug-loaded polymeric micelles; and (B) schematic representation of multiple effects of Pluronic block copolymers in MDR cells. Pluronic molecule binds with the cholesterol-rich domains in the cell membranes (“lipid-rafts”) (1) and perturbs their structure. This results in inhibition of the ATPase activity of the drug efflux pumps, Pgp and BCRP (2). Pluronic translocates into mitochondria, decreases mitochondria membrane potential and inhibits respiration (3). This leads to inhibition of the mitochondrial H+-ATPase and ATP depletion (4). The ATP depletion (4) along with inhibition of the ATPase activity of the Pgp and BCRP (2) results in the impairment of the drug efflux and increased accumulation of the drug in cells (5). The interaction of Pluronic in mitochondria also releases cytochrome C, increases ROS production, and shifts the cell signaling in response to the drug towards apoptosis (6). The Pgp inhibition, ATP depletion, accumulation of ROS and enhanced pro-apoptotic signaling vs. anti-apoptotic defense are usually observed in MDR cells but not in their sensitive counterparts. Based on references [17, 18, 239].
Similar articles
- Nanomedicine-mediated cancer stem cell therapy.
Shen S, Xia JX, Wang J. Shen S, et al. Biomaterials. 2016 Jan;74:1-18. doi: 10.1016/j.biomaterials.2015.09.037. Epub 2015 Sep 28. Biomaterials. 2016. PMID: 26433488 Review. - Eradicating the tumor "seeds": nanomedicines-based therapies against cancer stem cells.
Li L, Ni R, Zheng D, Chen L. Li L, et al. Daru. 2023 Jun;31(1):83-94. doi: 10.1007/s40199-023-00456-0. Epub 2023 Mar 27. Daru. 2023. PMID: 36971921 Free PMC article. Review. - Role of integrated cancer nanomedicine in overcoming drug resistance.
Iyer AK, Singh A, Ganta S, Amiji MM. Iyer AK, et al. Adv Drug Deliv Rev. 2013 Nov;65(13-14):1784-802. doi: 10.1016/j.addr.2013.07.012. Epub 2013 Jul 21. Adv Drug Deliv Rev. 2013. PMID: 23880506 Review. - Delivering cancer stem cell therapies - a role for nanomedicines?
Schätzlein AG. Schätzlein AG. Eur J Cancer. 2006 Jun;42(9):1309-15. doi: 10.1016/j.ejca.2006.01.044. Epub 2006 May 8. Eur J Cancer. 2006. PMID: 16682183 Review. - Reprogramming Cancer Stem-like Cells with Nanoforskolin Enhances the Efficacy of Paclitaxel in Targeting Breast Cancer.
Singh D, Singh P, Pradhan A, Srivastava R, Sahoo SK. Singh D, et al. ACS Appl Bio Mater. 2021 Apr 19;4(4):3670-3685. doi: 10.1021/acsabm.1c00141. Epub 2021 Apr 6. ACS Appl Bio Mater. 2021. PMID: 35014452
Cited by
- Star-Graft Quarterpolymer-Based Polymersomes as Nanocarriers for Co-Delivery of Hydrophilic/Hydrophobic Chemotherapeutic Agents.
Iatridi Z, Angelopoulou A, Voulgari E, Avgoustakis K, Tsitsilianis C. Iatridi Z, et al. ACS Omega. 2018 Sep 30;3(9):11896-11908. doi: 10.1021/acsomega.8b01437. Epub 2018 Sep 25. ACS Omega. 2018. PMID: 30320280 Free PMC article. - Synthesis of poly(1,2-glycerol carbonate)-paclitaxel conjugates and their utility as a single high-dose replacement for multi-dose treatment regimens in peritoneal cancer.
Ekladious I, Liu R, Zhang H, Foil DH, Todd DA, Graf TN, Padera RF, Oberlies NH, Colson YL, Grinstaff MW. Ekladious I, et al. Chem Sci. 2017 Dec 1;8(12):8443-8450. doi: 10.1039/c7sc03501b. Epub 2017 Oct 20. Chem Sci. 2017. PMID: 29619192 Free PMC article. - Aptamer-Based Therapeutic Approaches to Target Cancer Stem Cells.
Zhou G, Latchoumanin O, Bagdesar M, Hebbard L, Duan W, Liddle C, George J, Qiao L. Zhou G, et al. Theranostics. 2017 Sep 13;7(16):3948-3961. doi: 10.7150/thno.20725. eCollection 2017. Theranostics. 2017. PMID: 29109790 Free PMC article. Review. - Triple drugs co-delivered by a small gemcitabine-based carrier for pancreatic cancer immunochemotherapy.
Sun J, Wan Z, Chen Y, Xu J, Luo Z, Parise RA, Diao D, Ren P, Beumer JH, Lu B, Li S. Sun J, et al. Acta Biomater. 2020 Apr 1;106:289-300. doi: 10.1016/j.actbio.2020.01.039. Epub 2020 Jan 28. Acta Biomater. 2020. PMID: 32004652 Free PMC article. - Pluronics and MDR reversal: an update.
Alakhova DY, Kabanov AV. Alakhova DY, et al. Mol Pharm. 2014 Aug 4;11(8):2566-78. doi: 10.1021/mp500298q. Epub 2014 Jul 10. Mol Pharm. 2014. PMID: 24950236 Free PMC article. Review.
References
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nature medicine. 2009;15:1010–1012. - PubMed
- Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. - PubMed
- Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. - PubMed
- Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. - PubMed
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nature reviews. Cancer. 2005;5:275–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20GM103480/GM/NIGMS NIH HHS/United States
- U01 CA151806/CA/NCI NIH HHS/United States
- 2R01 CA89225/CA/NCI NIH HHS/United States
- UO1 CA151806/CA/NCI NIH HHS/United States
- P20 GM103480/GM/NIGMS NIH HHS/United States
- R01 CA089225/CA/NCI NIH HHS/United States
- P20 RR021937/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources