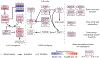Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation - PubMed (original) (raw)
. 2013 Dec;45(12):1459-63.
doi: 10.1038/ng.2798. Epub 2013 Oct 13.
Xiaojuan Sun, Chao Chen, Song Wu, Peide Huang, Zesong Li, Michael Dean, Yi Huang, Wenlong Jia, Quan Zhou, Aifa Tang, Zuoquan Yang, Xianxin Li, Pengfei Song, Xiaokun Zhao, Rui Ye, Shiqiang Zhang, Zhao Lin, Mingfu Qi, Shengqing Wan, Liangfu Xie, Fan Fan, Michael L Nickerson, Xiangjun Zou, Xueda Hu, Li Xing, Zhaojie Lv, Hongbin Mei, Shengjie Gao, Chaozhao Liang, Zhibo Gao, Jingxiao Lu, Yuan Yu, Chunxiao Liu, Lin Li, Xiaodong Fang, Zhimao Jiang, Jie Yang, Cailing Li, Xin Zhao, Jing Chen, Fang Zhang, Yongqi Lai, Zheguang Lin, Fangjian Zhou, Hao Chen, Hsiao Chang Chan, Shirley Tsang, Dan Theodorescu, Yingrui Li, Xiuqing Zhang, Jian Wang, Huanming Yang, Yaoting Gui, Jun Wang, Zhiming Cai
Affiliations
- PMID: 24121792
- PMCID: PMC7512009
- DOI: 10.1038/ng.2798
Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation
Guangwu Guo et al. Nat Genet. 2013 Dec.
Abstract
Bladder cancer is one of the most common cancers worldwide, with transitional cell carcinoma (TCC) being the predominant form. Here we report a genomic analysis of TCC by both whole-genome and whole-exome sequencing of 99 individuals with TCC. Beyond confirming recurrent mutations in genes previously identified as being mutated in TCC, we identified additional altered genes and pathways that were implicated in TCC. Notably, we discovered frequent alterations in STAG2 and ESPL1, two genes involved in the sister chromatid cohesion and segregation (SCCS) process. Furthermore, we also detected a recurrent fusion involving FGFR3 and TACC3, another component of SCCS, by transcriptome sequencing of 42 DNA-sequenced tumors. Overall, 32 of the 99 tumors (32%) harbored genetic alterations in the SCCS process. Our analysis provides evidence that genetic alterations affecting the SCCS process may be involved in bladder tumorigenesis and identifies a new therapeutic possibility for bladder cancer.
Figures
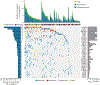
Figure 1
Significantly mutated genes in TCC as determined by exome sequencing. Significantly mutated genes are listed on the right. The percentage of bladder tumors with mutations detected by automated calling is noted on the left. The upper histogram shows the somatic mutation rate in each of the 99 tumors. The central heatmap shows the distribution of mutations across the sequenced samples. All the mutations shown were confirmed by Sanger sequencing. Genes with asterisks had mutations newly observed in TCC in this study.
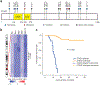
Figure 2
STAG2 somatic mutations and copy number changes in TCC. (a) Somatic alterations overlaid on the STAG2 protein with the conserved protein domains highlighted. STAG, STAG domain; SCD, stromalin conservative domain. (b) Five tumors harboring genomic deletions of STAG2 on the X chromosome (ideogram shown on the left). (c) Kaplan-Meier survival analysis of individuals with TCC (n = 99) shows that the survival rate for individuals with somatic STAG2 alterations (n = 16) is significantly lower (log-rank test P < 0.001) than for individuals with wild-type STAG2 (n = 83).
Figure 3
FGFR3-TACC3 fusion was identified in TCC. (a) Genomic fusion of intron 17 of FGFR3 with intron 10 of TACC3 resulting in exon 17 of FGFR3 being spliced 5’ to exon 11 of TACC3 in the fused mRNA. Triangles indicate the genomic positions of the breakpoints. Detailed information on the positions and sequences of primers P1 and P3 is provided in Supplementary Table 14. (b) Outlier high expression of TACC3 in TCCs harboring FGFR3-TACC3 gene fusions. RPKM, reads per kilobase of exon region in a gene per million mapped reads, (c) RNA-seq coverage analysis of FGFR3 (top) and TACC3 (bottom) in the tumor and matched normal bladder tissue from B59-3. Three transcripts of FGFR3 and one transcript of TACC3 are shown. Black dotted lines indicate breakpoints. E, exon.
Figure 4
Frequent genetic alterations in genes from the cell cycle pathway in TCC. Alterations are defined as somatic mutations, focal amplifications and deletions, and, in some cases, as gene fusion events. Alteration frequencies are expressed as a percentage of all cases.
Comment in
- Bladder cancer: STAG2 in the spotlight--have genomic studies identified a plausible biomarker?
Razzak M. Razzak M. Nat Rev Urol. 2013 Dec;10(12):675. doi: 10.1038/nrurol.2013.253. Epub 2013 Nov 5. Nat Rev Urol. 2013. PMID: 24189933 No abstract available. - Frequent truncating mutations of STAG2 in bladder cancer.
Black P. Black P. Urology. 2014 Apr;83(4):691-2. doi: 10.1016/j.urology.2013.11.027. Epub 2014 Jan 31. Urology. 2014. PMID: 24486001 No abstract available.
Similar articles
- Frequent truncating mutations of STAG2 in bladder cancer.
Black P. Black P. Urology. 2014 Apr;83(4):691-2. doi: 10.1016/j.urology.2013.11.027. Epub 2014 Jan 31. Urology. 2014. PMID: 24486001 No abstract available. - Bladder cancer: STAG2 in the spotlight--have genomic studies identified a plausible biomarker?
Razzak M. Razzak M. Nat Rev Urol. 2013 Dec;10(12):675. doi: 10.1038/nrurol.2013.253. Epub 2013 Nov 5. Nat Rev Urol. 2013. PMID: 24189933 No abstract available. - Genetic and molecular markers of urothelial premalignancy and malignancy.
Cordon-Cardo C, Cote RJ, Sauter G. Cordon-Cardo C, et al. Scand J Urol Nephrol Suppl. 2000;(205):82-93. doi: 10.1080/003655900750169338. Scand J Urol Nephrol Suppl. 2000. PMID: 11144907 Review. - Sister chromatid cohesion and recombination in meiosis.
van Heemst D, Heyting C. van Heemst D, et al. Chromosoma. 2000;109(1-2):10-26. doi: 10.1007/s004120050408. Chromosoma. 2000. PMID: 10855491 Review.
Cited by
- HER2 as a target in invasive urothelial carcinoma.
Bellmunt J, Werner L, Bamias A, Fay AP, Park RS, Riester M, Selvarajah S, Barletta JA, Berman DM, de Muga S, Salido M, Gallardo E, Rojo F, Guancial EA, Bambury R, Mullane SA, Choueiri TK, Loda M, Stack E, Rosenberg J. Bellmunt J, et al. Cancer Med. 2015 Jun;4(6):844-52. doi: 10.1002/cam4.432. Epub 2015 Feb 26. Cancer Med. 2015. PMID: 25720673 Free PMC article. - Oncogenic mutations and dysregulated pathways in obesity-associated hepatocellular carcinoma.
Shen J, Tsoi H, Liang Q, Chu ES, Liu D, Yu AC, Chan TF, Li X, Sung JJ, Wong VW, Yu J. Shen J, et al. Oncogene. 2016 Dec 8;35(49):6271-6280. doi: 10.1038/onc.2016.162. Epub 2016 May 2. Oncogene. 2016. PMID: 27132506 Free PMC article. - Evolution of Urothelial Bladder Cancer in the Context of Molecular Classifications.
Minoli M, Kiener M, Thalmann GN, Kruithof-de Julio M, Seiler R. Minoli M, et al. Int J Mol Sci. 2020 Aug 7;21(16):5670. doi: 10.3390/ijms21165670. Int J Mol Sci. 2020. PMID: 32784716 Free PMC article. Review. - Next generation sequencing and urologic cancer.
Kim SK, Kim WJ. Kim SK, et al. Korean J Urol. 2015 Feb;56(2):87-9. doi: 10.4111/kju.2015.56.2.87. Korean J Urol. 2015. PMID: 25685294 Free PMC article. No abstract available. - The Interplay of Cohesin and RNA Processing Factors: The Impact of Their Alterations on Genome Stability.
Osadska M, Selicky T, Kretova M, Jurcik J, Sivakova B, Cipakova I, Cipak L. Osadska M, et al. Int J Mol Sci. 2022 Apr 1;23(7):3939. doi: 10.3390/ijms23073939. Int J Mol Sci. 2022. PMID: 35409298 Free PMC article. Review.
References
- Jemal A et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011). - PubMed
- Wu XR Urothelial tumorigenesis: a tale of divergent pathways. Nat. Rev. Cancer 5, 713–725 (2005). - PubMed
- Cordon-Cardo C et al. p53 mutations in human bladder cancer: genotypic versus phenotypic patterns. Int. J. Cancer 56, 347–353 (1994). - PubMed
- Jebar AH et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 24, 5218–5225 (2005). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical