TLRs control hematopoiesis during infection - PubMed (original) (raw)
Review
TLRs control hematopoiesis during infection
Alberto Yáñez et al. Eur J Immunol. 2013 Oct.
Abstract
Recent research has shown that (i) Toll-like receptor (TLR) agonists drive hematopoietic stem and progenitor cells (HSPCs) to proliferate and differentiate along the myeloid lineage in vitro, and (ii) direct TLR-mediated stimulation of HSPCs also promotes macrophage differentiation in vivo following infection. These new insights demonstrate that TLR signaling in HSPCs, in addition to other TLR-dependent mechanisms, can contribute to HSPC expansion and myeloid differentiation after infection. Evidence is, therefore, mounting that direct TLR-induced programming of hematopoiesis plays a key role in host defense by rapidly replenishing the innate immune system with the cells needed to deal with pathogens.
Keywords: Hematopoiesis; Hematopoietic stem and progenitor cells; Infection; TLRs.
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Figures
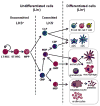
Figure 1. The mouse hematopoietic tree
Hematopoiesis is initiated in the bone marrow by normally quiescent long-term hematopoietic stem cells (LT-HSCs), which have the capacity for self-renewal and give rise to proliferating short-term HSCs (ST-HSCs). ST-HSCs produce multipotent progenitors (MPPs), which give rise to progenitors committed to specific hematopoietic lineages, common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs). Mouse HSPCs from the bone marrow are defined by their lack of expression of markers of differentiated cells. A cocktail of antibodies specific for differentiated blood cell antigens, termed “lineage markers” (Lin; typically CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), 7–4, and Ter-119) can be used to eliminate mature hematopoietic lineages. The remaining Lin− cells can then be enriched for specific stem/progenitor populations. Sorting Lin− c-Kit+ Sca-1+ (LKS+) cells enriches for hematopoietic-reconstituting activity (i.e. HSCs). However, only 10% LKS+ cells are bona fide long-term reconstituting (LT)-HSCs; the LKS+ population also includes short-term (ST)-HSCs and MPPs. The Lin− c-Kit+ Sca-1− (LKS−) fraction contains oligopotent lineage-committed progenitors. CLPs generate all classes of lymphocytes, and CMPs give rise to either megakaryocyte-erythrocyte progenitors (MEPs) or granulocyte-monocyte progenitors (GMPs). Dendritic cell (DC) potential is retained in both CMPs and CLPs. In addition to producing GMPs, CMPs also give rise to monocyte and DC progenitors (MDPs), which can generate monocytes, macrophages, classical DCs (cDCs) and plasmacytoid DCs (pDCs). MDPs lie upstream of the common DC progenitors (CDPs), which are DC-restricted, giving rise to pDCs and cDCs. Monocytes can further differentiate into macrophages or monocyte-derived DCs (moDCs).
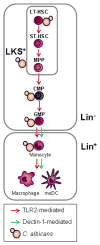
Figure 2. C. albicans directly stimulates HSPCs to induce myelopoiesis
C. albicans interacts in vitro with mouse HSPCs from the most quiescent HSCs (LT-HSCs) to the lineage-committed progenitors (CMPs and GMPs, top), inducing the differentiation of these cells towards the myeloid lineage in a TLR2-dependent manner. C. albicans also induces TLR2- and Dectin-1-dependent production of moDCs by Lin− HSPCs in vitro, and TLR2-dependent macrophage production by transplanted LKS+ and Lin− cells upon C. albicans infection in vivo (bottom). Dectin-1 activation also promotes monocyte differentiation to macrophages and moDCs (bottom).
Similar articles
- Dectin-1 Stimulation of Hematopoietic Stem and Progenitor Cells Occurs In Vivo and Promotes Differentiation Toward Trained Macrophages via an Indirect Cell-Autonomous Mechanism.
Bono C, Martínez A, Megías J, Gozalbo D, Yáñez A, Gil ML. Bono C, et al. mBio. 2020 Jun 23;11(3):e00781-20. doi: 10.1128/mBio.00781-20. mBio. 2020. PMID: 32576672 Free PMC article. - Deconstructing innate immune signaling in myelodysplastic syndromes.
Varney ME, Melgar K, Niederkorn M, Smith M, Barreyro L, Starczynowski DT. Varney ME, et al. Exp Hematol. 2015 Aug;43(8):587-598. doi: 10.1016/j.exphem.2015.05.016. Epub 2015 Jul 2. Exp Hematol. 2015. PMID: 26143580 Free PMC article. Review. - Microbial sensing by haematopoietic stem and progenitor cells: Vigilance against infections and immune education of myeloid cells.
Sioud M. Sioud M. Scand J Immunol. 2020 Nov;92(5):e12957. doi: 10.1111/sji.12957. Epub 2020 Oct 7. Scand J Immunol. 2020. PMID: 32767789 Review. - Direct Toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages.
Megías J, Yáñez A, Moriano S, O'Connor JE, Gozalbo D, Gil ML. Megías J, et al. Stem Cells. 2012 Jul;30(7):1486-95. doi: 10.1002/stem.1110. Stem Cells. 2012. PMID: 22511319 - The Friend of GATA Transcriptional Co-Regulator, U-Shaped, Is a Downstream Antagonist of Dorsal-Driven Prohemocyte Differentiation in Drosophila.
Gao H, Baldeosingh R, Wu X, Fossett N. Gao H, et al. PLoS One. 2016 May 10;11(5):e0155372. doi: 10.1371/journal.pone.0155372. eCollection 2016. PLoS One. 2016. PMID: 27163255 Free PMC article.
Cited by
- The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies.
Wiertsema SP, van Bergenhenegouwen J, Garssen J, Knippels LMJ. Wiertsema SP, et al. Nutrients. 2021 Mar 9;13(3):886. doi: 10.3390/nu13030886. Nutrients. 2021. PMID: 33803407 Free PMC article. Review. - Non-haematopoietic Sca-1+ Cells in the Retina of Adult Mice Express Functional TLR2.
Flores A, Fernández-Sánchez L, Kutsyr O, Lax P, Yáñez A, Gil ML, Gozalbo D, Maneu V. Flores A, et al. Stem Cell Rev Rep. 2024 Apr;20(3):845-851. doi: 10.1007/s12015-023-10674-3. Epub 2024 Jan 6. Stem Cell Rev Rep. 2024. PMID: 38183535 - SARS-CoV-2 infection augments species- and age-specific predispositions in cotton rats.
Boukhvalova MS, Mortensen E, Caple J, Joseph J, Sylla F, Kamali A, Stylos D, Lopez D, March T, Byrd KM, Prince GA, Arndt A, Kajon A, Blanco JCG. Boukhvalova MS, et al. Sci Rep. 2023 Jan 14;13(1):757. doi: 10.1038/s41598-022-27328-y. Sci Rep. 2023. PMID: 36641520 Free PMC article. - Hematopoietic stem and progenitor cells directly participate in host immune response.
Daramola OJ, Osasan S, Ali H, Emeagi P. Daramola OJ, et al. Am J Stem Cells. 2021 Jun 15;10(2):18-27. eCollection 2021. Am J Stem Cells. 2021. PMID: 34327049 Free PMC article. Review. - Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes.
Yáñez A, Coetzee SG, Olsson A, Muench DE, Berman BP, Hazelett DJ, Salomonis N, Grimes HL, Goodridge HS. Yáñez A, et al. Immunity. 2017 Nov 21;47(5):890-902.e4. doi: 10.1016/j.immuni.2017.10.021. Immunity. 2017. PMID: 29166589 Free PMC article.
References
- Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev. 2010;234:32–44. - PubMed
- Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical