Placebo improves pleasure and pain through opposite modulation of sensory processing - PubMed (original) (raw)
Clinical Trial
. 2013 Oct 29;110(44):17993-8.
doi: 10.1073/pnas.1305050110. Epub 2013 Oct 14.
Affiliations
- PMID: 24127578
- PMCID: PMC3816412
- DOI: 10.1073/pnas.1305050110
Clinical Trial
Placebo improves pleasure and pain through opposite modulation of sensory processing
Dan-Mikael Ellingsen et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Placebo analgesia is often conceptualized as a reward mechanism. However, by targeting only negative experiences, such as pain, placebo research may tell only half the story. We compared placebo improvement of painful touch (analgesia) with placebo improvement of pleasant touch (hyperhedonia) using functional MRI and a crossover design. Somatosensory processing was decreased during placebo analgesia and increased during placebo hyperhedonia. Both placebo responses were associated with similar patterns of activation increase in circuitry involved in emotion appraisal, including the pregenual anterior cingulate, medial orbitofrontal cortex, amygdala, accumbens, and midbrain structures. Importantly, placebo-induced coupling between the ventromedial prefrontal cortex and periaqueductal gray correlated with somatosensory decreases to painful touch and somatosensory increases to pleasant touch. These findings suggest that placebo analgesia and hyperhedonia are mediated by activation of shared emotion appraisal neurocircuitry, which down- or up-regulates early sensory processing, depending on whether the expectation is reduced pain or increased pleasure.
Keywords: expectancy; hedonic feelings; neuroimaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
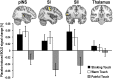
Behavioral results. (A) After watching the documentary, participants indicated a positive expectation that intranasal oxytocin treatment would induce stroking touch and warm touch hyperhedonia, as well as analgesia, but no expectation of oxytocin effects on irrelevant control statements. (B) Compared with the control condition, placebo treatment increased pleasantness of stroking and warm touch, and decreased unpleasantness of painful touch. (C) The magnitude of placebo responses [defined as the (placebo > control) difference in VAS scores] correlated across stimulus types. Error bars represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 2.
Placebo-induced BOLD responses in somatosensory circuitry. Placebo improvement of painful and pleasant touch experiences was underpinned by opposite BOLD effects in contralateral somatosensory areas (pINS, SI, and SII). After placebo treatment, BOLD responses to pleasant touch were increased, but BOLD responses to painful touch were decreased. Averaged activation maps [Z > 2, uncorrected for illustration purposes, superimposed on the Montreal Neurological Institute (MNI) standard template brain] show voxels where placebo-induced BOLD changes during stroking touch (green) and warm touch (yellow) were significantly more positive than during painful touch (orange represents overlap between stroking and warm touch). Averaged percent signal change values (placebo > control) from the ROIs (bottom) are plotted for illustration purposes. Error bars represent SEM.
Fig. 3.
Placebo-induced BOLD responses in a priori-defined emotion appraisal neurocircuitry. (A) The group contrast (placebo > control) revealed overlapping placebo-induced BOLD increases in the NAc during stroking, warm, and painful touch (as revealed by conjunction analysis), and in the PAG during stroking and warm touch. (B) Regions where individual placebo response (placebo > control) correlated with placebo-induced (placebo > control) BOLD increase. High placebo responses correlated with high placebo-induced increases in these regions. (C) Magnitude of stroking touch hyperhedonia correlated with increased functional coupling between the mOFC, left NAc, left amygdala, and the PAG. Magnitude of placebo analgesia correlated with increased functional coupling between pgACC and mOFC, and bilateral amygdalae as well as mesolimbic reward regions (right NAc and VTA). Green represents stroking touch; yellow represents warm touch; red represents painful touch. Averaged activation maps (Z > 2, uncorrected for illustration purposes) were superimposed on the MNI standard template brain.
Fig. 4.
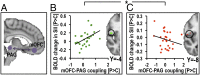
mOFC–PAG coupling strength was associated with opposite modulation of SII in placebo analgesia and hyperhedonia. Strong placebo-induced functional coupling between mOFC and PAG (A) correlated with increased SII responses to stroking touch (B) but decreased SII responses to painful touch (C), a pattern that was replicated also for the pINS. Averaged activation maps were thresholded at Z > 2, uncorrected, for illustrational purposes. The scatterplots illustrate the correlations, which are significantly different from each other. *P > 0.05.
Fig. 5.
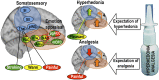
Proposed mechanism of placebo analgesia and hyperhedonia. During expectation of hyperhedonia and analgesia, a shared modulatory network up-regulates pleasant touch processing and down-regulates painful touch processing in somatosensory areas, possibly through similar dopaminergic/opioidergic connections. Color-coding of the regions represent areas where placebo treatment induced activation for stroking touch (green), warm touch (yellow), and painful touch (red). Connecting lines represent placebo-related increases in functional connectivity for stroking touch (green) and painful touch (red). Somatosensory regions are shown in blue.
Similar articles
- A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions.
Atlas LY, Wager TD. Atlas LY, et al. Handb Exp Pharmacol. 2014;225:37-69. doi: 10.1007/978-3-662-44519-8_3. Handb Exp Pharmacol. 2014. PMID: 25304525 Free PMC article. - Neural bases of conditioned placebo analgesia.
Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. Lui F, et al. Pain. 2010 Dec;151(3):816-824. doi: 10.1016/j.pain.2010.09.021. Epub 2010 Oct 12. Pain. 2010. PMID: 20943318 - The importance of context: when relative relief renders pain pleasant.
Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I. Leknes S, et al. Pain. 2013 Mar;154(3):402-410. doi: 10.1016/j.pain.2012.11.018. Epub 2012 Dec 13. Pain. 2013. PMID: 23352758 Free PMC article. Clinical Trial. - Placebo analgesia: findings from brain imaging studies and emerging hypotheses.
Kong J, Kaptchuk TJ, Polich G, Kirsch I, Gollub RL. Kong J, et al. Rev Neurosci. 2007;18(3-4):173-90. doi: 10.1515/revneuro.2007.18.3-4.173. Rev Neurosci. 2007. PMID: 18019605 Review. - Neuroimaging study of placebo analgesia in humans.
Qiu YH, Wu XY, Xu H, Sackett D. Qiu YH, et al. Neurosci Bull. 2009 Oct;25(5):277-82. doi: 10.1007/s12264-009-0907-2. Neurosci Bull. 2009. PMID: 19784082 Free PMC article. Review.
Cited by
- Top-Down Dysregulation-From ADHD to Emotional Instability.
Petrovic P, Castellanos FX. Petrovic P, et al. Front Behav Neurosci. 2016 May 23;10:70. doi: 10.3389/fnbeh.2016.00070. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27242456 Free PMC article. - Therapeutic Basis of Clinical Pain Modulation.
Kirkpatrick DR, McEntire DM, Hambsch ZJ, Kerfeld MJ, Smith TA, Reisbig MD, Youngblood CF, Agrawal DK. Kirkpatrick DR, et al. Clin Transl Sci. 2015 Dec;8(6):848-56. doi: 10.1111/cts.12282. Epub 2015 May 11. Clin Transl Sci. 2015. PMID: 25962969 Free PMC article. Review. - Placebo Effects on the Neurologic Pain Signature: A Meta-analysis of Individual Participant Functional Magnetic Resonance Imaging Data.
Zunhammer M, Bingel U, Wager TD; Placebo Imaging Consortium. Zunhammer M, et al. JAMA Neurol. 2018 Nov 1;75(11):1321-1330. doi: 10.1001/jamaneurol.2018.2017. JAMA Neurol. 2018. PMID: 30073258 Free PMC article. - ALE meta-analysis reveals dissociable networks for affective and discriminative aspects of touch.
Morrison I. Morrison I. Hum Brain Mapp. 2016 Apr;37(4):1308-20. doi: 10.1002/hbm.23103. Epub 2016 Feb 1. Hum Brain Mapp. 2016. PMID: 26873519 Free PMC article. - The relation between human hair follicle density and touch perception.
Jönsson EH, Bendas J, Weidner K, Wessberg J, Olausson H, Wasling HB, Croy I. Jönsson EH, et al. Sci Rep. 2017 May 31;7(1):2499. doi: 10.1038/s41598-017-02308-9. Sci Rep. 2017. PMID: 28566678 Free PMC article.
References
- Leknes S, Brooks JC, Wiech K, Tracey I. Pain relief as an opponent process: A psychophysical investigation. Eur J Neurosci. 2008;28(4):794–801. - PubMed
- Seymour B, et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8(9):1234–1240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical