Large extent of disorder in Adenomatous Polyposis Coli offers a strategy to guard Wnt signalling against point mutations - PubMed (original) (raw)
Large extent of disorder in Adenomatous Polyposis Coli offers a strategy to guard Wnt signalling against point mutations
David P Minde et al. PLoS One. 2013.
Abstract
Mutations in the central region of the signalling hub Adenomatous Polyposis Coli (APC) cause colorectal tumourigenesis. The structure of this region remained unknown. Here, we characterise the Mutation Cluster Region in APC (APC-MCR) as intrinsically disordered and propose a model how this structural feature may contribute to regulation of Wnt signalling by phosphorylation. APC-MCR was susceptible to proteolysis, lacked α-helical secondary structure and did not display thermal unfolding transition. It displayed an extended conformation in size exclusion chromatography and was accessible for phosphorylation by CK1ε in vitro. The length of disordered regions in APC increases with species complexity, from C. elegans to H. sapiens. We speculate that the large disordered region harbouring phosphorylation sites could be a successful strategy to stabilise tight regulation of Wnt signalling against single missense mutations.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
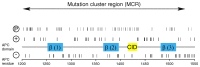
Figure 1. Charge distribution of APC-MCR.
Charges present in the primary structure, putative phosphorylation sites and regulatory important motifs are indicated. A large number of putative phosphorylation sites scatter over the entire MCR (Phosida predictor for S/T phosphorylation). Two of the three 20 amino acid repeats (blue), bind to β-catenin (β). A short peptide stretch called “CID” (yellow) was recently implicated in Wnt/β-catenin downregulation [4,70].
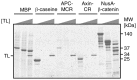
Figure 2. MCR is susceptible to TL digestion.
Resistance of well-characterised folded and unfolded proteins is compared with MCR by using TL concentrations of 0 g/L, 0.001 g/L and 0.1 g/L. Folded protein MBP (diamond), is resistant against the highest protease concentration while β-caseine and Axin CR are already cleaved at low protease concentration (0.001g/L). A fusion construct of NusA and β-catenin gives several high molecular weight bands, likely due to the presence of at least one large protease susceptible internal linker segment [65].
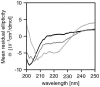
Figure 3. MCR lacks secondary structure.
Far-UV CD spectra of APC-MCR in the absence (black) and presence of TFE (dark grey, 20%; light grey, 80%) in 10 mM Na-phosphate buffer (pH 7.2), 50 mM NaF, and 0.5 mM TCEP.
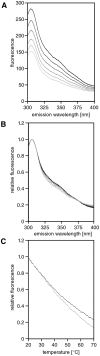
Figure 4. APC-MCR lacks a cooperative unfolding transition.
A, Intrinsic protein fluorescence spectra of APC-MCR measured in 1°C steps from 20°C to 70°C, indicated by a greyscale gradient from black to light grey**. B**, Comparison of intrinsic tyrosine fluorescence spectra normalised to the maxima. Experiment and colour code as in A. C Temperature dependence of intrinsic tyrosine fluorescence emission of MCR upon stepwise increase of temperature at 340 nm (black) and 304 nm (grey) normalised on maxima of emission.
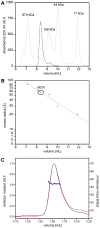
Figure 5. APC-MCR runs at a higher apparent molecular weight upon size exclusion chromatography.
A, APC-MCR was applied to analytical size exclusion chromatography. MCR eluted after 8.36 min between the indicated globular size standards. By semilogarithmic fitting to the molecular weights of the standards, an apparent molecular weight of 220 kDa has been determined for APC-MCR. B, The straight line indicates a semilogarithmic fit to the known stokes radii of the indicated standard proteins. According to this analysis, MCR has a stokes radius of 5.9 nm. C, SEC-MALLS reveals that the ySUMO-APC-MCR fusion protein is monomeric. ySUMO-APC-MCR eluted as a single peak with a mass of 44.4 ± 4.0 kDa (UV absorption at 220 nm, black, arbitrary units; differential refractive index, red, arbitrary units; Molecular mass, blue, kDa).
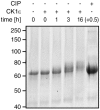
Figure 6. MCR can be phosphorylated by CK1ε.
ySUMO-MCR was incubated for the times indicated with CK1ε. The bandshift of phosphorylated ySUMO-MCR after 16 h phosphorylation was fully reversed by heat inactivation of CK1ε followed by dephosphorylation with CIP for 30 min (last lane).
Figure 7. The three Wnt signalling hubs APC, Axin, WTX contain large intrinsically disordered regions.
A, Meta-predictions of disorder using the PONDR-FIT algorithm are displayed. (black for scores >= 0.5 and white for scores < 0.5). B, Same as A, for C. elegans homologues of APC, Axin, Hsp90, CBP.
Similar articles
- The 15-Amino Acid Repeat Region of Adenomatous Polyposis Coli Is Intrinsically Disordered and Retains Conformational Flexibility upon Binding β-Catenin.
Rudeen AJ, Douglas JT, Xing M, McDonald WH, Lamb AL, Neufeld KL. Rudeen AJ, et al. Biochemistry. 2020 Oct 20;59(41):4039-4050. doi: 10.1021/acs.biochem.0c00479. Epub 2020 Oct 1. Biochemistry. 2020. PMID: 32941008 Free PMC article. - A targeted constitutive mutation in the APC tumor suppressor gene underlies mammary but not intestinal tumorigenesis.
Gaspar C, Franken P, Molenaar L, Breukel C, van der Valk M, Smits R, Fodde R. Gaspar C, et al. PLoS Genet. 2009 Jul;5(7):e1000547. doi: 10.1371/journal.pgen.1000547. Epub 2009 Jul 3. PLoS Genet. 2009. PMID: 19578404 Free PMC article. - Adenomatous polyposis coli (APC) membrane recruitment 3, a member of the APC membrane recruitment family of APC-binding proteins, is a positive regulator of Wnt-β-catenin signalling.
Brauburger K, Akyildiz S, Ruppert JG, Graeb M, Bernkopf DB, Hadjihannas MV, Behrens J. Brauburger K, et al. FEBS J. 2014 Feb;281(3):787-801. doi: 10.1111/febs.12624. Epub 2013 Dec 12. FEBS J. 2014. PMID: 24251807 - The adenomatous polyposis coli protein 30 years on.
Abbott J, Näthke IS. Abbott J, et al. Semin Cell Dev Biol. 2023 Dec;150-151:28-34. doi: 10.1016/j.semcdb.2023.04.004. Epub 2023 Apr 22. Semin Cell Dev Biol. 2023. PMID: 37095033 Review. - APC.
Bienz M. Bienz M. Curr Biol. 2003 Mar 18;13(6):R215-6. doi: 10.1016/s0960-9822(03)00152-0. Curr Biol. 2003. PMID: 12646143 Review. No abstract available.
Cited by
- Targeting cancer stem cells in drug discovery: Current state and future perspectives.
Du FY, Zhou QF, Sun WJ, Chen GL. Du FY, et al. World J Stem Cells. 2019 Jul 26;11(7):398-420. doi: 10.4252/wjsc.v11.i7.398. World J Stem Cells. 2019. PMID: 31396368 Free PMC article. Review. - Construing the Biochemical and Molecular Mechanism Underlying the In Vivo and In Vitro Chemotherapeutic Efficacy of Ruthenium-Baicalein Complex in Colon Cancer.
Wang Y, Bian L, Chakraborty T, Ghosh T, Chanda P, Roy S. Wang Y, et al. Int J Biol Sci. 2019 Apr 22;15(5):1052-1071. doi: 10.7150/ijbs.31143. eCollection 2019. Int J Biol Sci. 2019. PMID: 31182925 Free PMC article. - Designing disorder: Tales of the unexpected tails.
Minde DP, Halff EF, Tans S. Minde DP, et al. Intrinsically Disord Proteins. 2013 Jan 1;1(1):e26790. doi: 10.4161/idp.26790. eCollection 2013 Jan-Dec. Intrinsically Disord Proteins. 2013. PMID: 28516025 Free PMC article. Review. - WNT Signaling in Disease.
Ng LF, Kaur P, Bunnag N, Suresh J, Sung ICH, Tan QH, Gruber J, Tolwinski NS. Ng LF, et al. Cells. 2019 Aug 3;8(8):826. doi: 10.3390/cells8080826. Cells. 2019. PMID: 31382613 Free PMC article. Review. - The role of TGF-β/SMAD4 signaling in cancer.
Zhao M, Mishra L, Deng CX. Zhao M, et al. Int J Biol Sci. 2018 Jan 12;14(2):111-123. doi: 10.7150/ijbs.23230. eCollection 2018. Int J Biol Sci. 2018. PMID: 29483830 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
SGDR was supported by the European Union's Sixth Framework Programme (FP6) by a Marie-Curie Excellence Grant (MEXT-CT-2005-025651) and by the Seventh Framework Programme (FP7) under grant agreement ManiFold n°317371, by a VIDI career development grant (700.55.421) by the Netherlands Organization for Scientific Research (NWO) and by a High Potential Grant of Utrecht University. MMM was supported by the European Research Council (ERC-StG no.242958) and by a High Potential Grant of Utrecht University. URLs of funders: NWO: http://www.nwo.nl/; EU: http://europa.eu/index_en.htm; Utrecht University: http://www.uu.nl/; ERC: http://erc.europa.eu/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources