Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2014 Feb;133(2):405-13.
doi: 10.1016/j.jaci.2013.08.020. Epub 2013 Oct 13.
Affiliations
- PMID: 24131826
- PMCID: PMC7112326
- DOI: 10.1016/j.jaci.2013.08.020
Randomized Controlled Trial
Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial
Raakel Luoto et al. J Allergy Clin Immunol. 2014 Feb.
Abstract
Background: Simple and safe strategies for the prevention of viral respiratory tract infections (RTIs) are needed.
Objective: We hypothesized that early prebiotic or probiotic supplementation would reduce the risk of virus-associated RTIs during the first year of life in a cohort of preterm infants.
Methods: In this randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov no. NCT00167700), 94 preterm infants (gestational age, ≥32 + 0 and ≤36 + 6 weeks; birth weight, >1500 g) treated at Turku University Hospital, Turku, Finland, were allocated to receive oral prebiotics (galacto-oligosaccharide and polydextrose mixture, 1:1), a probiotic (Lactobacillus rhamnosus GG, ATCC 53103), or placebo (microcrystalline cellulose) between days 3 and 60 of life. The primary outcome was the incidence of clinically defined virus-associated RTI episodes confirmed from nasal swabs by using nucleic acid testing. Secondary outcomes were the severity and duration of RTIs.
Results: A significantly lower incidence of RTIs was detected in infants receiving prebiotics (rate ratio [RR], 0.24; 95% CI, 0.12-0.49; P < .001) or probiotics (RR, 0.50; 95% CI, 0.28-0.90; P = .022) compared with those receiving placebo. Also, the incidence of rhinovirus-induced episodes, which comprised 80% of all RTI episodes, was found to be significantly lower in the prebiotic (RR, 0.31; 95% CI, 0.14-0.66; P = .003) and probiotic (RR, 0.49; 95% CI, 0.24-1.00; P = .051) groups compared with the placebo group. No differences emerged among the study groups in rhinovirus RNA load during infections, duration of rhinovirus RNA shedding, duration or severity of rhinovirus infections, or occurrence of rhinovirus RNA in asymptomatic infants.
Conclusions: Gut microbiota modification with specific prebiotics and probiotics might offer a novel and cost-effective means to reduce the risk of rhinovirus infections.
Keywords: Galacto-oligosaccharide; Lactobacillus rhamnosus GG; RR; RSV; RTI; Rate ratio; Respiratory syncytial virus; Respiratory tract infection; gut microbiota; polydextrose; prebiotic; preterm infant; probiotic; respiratory tract infections; rhinovirus.
Copyright © 2013 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures
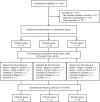
Fig 1
Trial flow of patients.
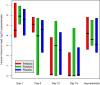
Fig 2
Median (range) rhinovirus load (log10 copies per sample) obtained when respiratory symptoms were present on days 1, 5, 10, and 15 of the episodes and in asymptomatic infants in the 3 study groups. No significant differences were detected among the study groups at any time point (P = .650 on day 1, P = .605 on day 5, P = .856 on day 10, P = .121 on day 15, and P = .990 in asymptomatic infants). The difference between symptomatic infants on day 1 and asymptomatic infants was significant (P < .001).
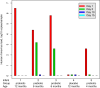
Fig 3
Rhinovirus load (log10 copies per sample) obtained in the 5 infants with asymptomatic rhinovirus-positive findings. Rhinovirus quantitative RT-PCR was performed on days 5, 10, and 15 after a verified asymptomatic rhinovirus RNA finding (day 1) to determine the duration of viral shedding.
Similar articles
- Effects of early prebiotic and probiotic supplementation on development of gut microbiota and fussing and crying in preterm infants: a randomized, double-blind, placebo-controlled trial.
Pärtty A, Luoto R, Kalliomäki M, Salminen S, Isolauri E. Pärtty A, et al. J Pediatr. 2013 Nov;163(5):1272-7.e1-2. doi: 10.1016/j.jpeds.2013.05.035. Epub 2013 Jul 31. J Pediatr. 2013. PMID: 23915796 Clinical Trial. - Reversible aberrancies in gut microbiome of moderate and late preterm infants: results from a randomized, controlled trial.
Luoto R, Pärtty A, Vogt JK, Rautava S, Isolauri E. Luoto R, et al. Gut Microbes. 2023 Dec;15(2):2283913. doi: 10.1080/19490976.2023.2283913. Epub 2023 Nov 27. Gut Microbes. 2023. PMID: 38010080 Free PMC article. Clinical Trial. - Human rhinovirus in experimental infection after peroral Lactobacillus rhamnosus GG consumption, a pilot study.
Tapiovaara L, Kumpu M, Mäkivuokko H, Waris M, Korpela R, Pitkäranta A, Winther B. Tapiovaara L, et al. Int Forum Allergy Rhinol. 2016 Aug;6(8):848-53. doi: 10.1002/alr.21748. Epub 2016 Mar 17. Int Forum Allergy Rhinol. 2016. PMID: 26990147 Clinical Trial. - Orally Ingested Probiotics, Prebiotics, and Synbiotics as Countermeasures for Respiratory Tract Infections in Nonelderly Adults: A Systematic Review and Meta-Analysis.
Coleman JL, Hatch-McChesney A, Small SD, Allen JT, Sullo E, Agans RT, Fagnant HS, Bukhari AS, Karl JP. Coleman JL, et al. Adv Nutr. 2022 Dec 22;13(6):2277-2295. doi: 10.1093/advances/nmac086. Adv Nutr. 2022. PMID: 35948276 Free PMC article. - Effect of prebiotic and probiotic supplementation on neurodevelopment in preterm very low birth weight infants: findings from a meta-analysis.
Upadhyay RP, Taneja S, Chowdhury R, Strand TA, Bhandari N. Upadhyay RP, et al. Pediatr Res. 2020 Apr;87(5):811-822. doi: 10.1038/s41390-018-0211-9. Epub 2018 Oct 18. Pediatr Res. 2020. PMID: 30353041 Review.
Cited by
- Probiotics potentials in mitigating coronavirus disease (COVID-19) pandemic.
Reuben RC, Makut MD, Adogo LY. Reuben RC, et al. Pan Afr Med J. 2021 Feb 18;38:186. doi: 10.11604/pamj.2021.38.186.27953. eCollection 2021. Pan Afr Med J. 2021. PMID: 33995792 Free PMC article. - The Microbiome and Sustainable Healthcare.
Dietert RR, Dietert JM. Dietert RR, et al. Healthcare (Basel). 2015 Mar 3;3(1):100-29. doi: 10.3390/healthcare3010100. Healthcare (Basel). 2015. PMID: 27417751 Free PMC article. Review. - The Beneficial Role of Probiotic Lactobacillus in Respiratory Diseases.
Du T, Lei A, Zhang N, Zhu C. Du T, et al. Front Immunol. 2022 May 31;13:908010. doi: 10.3389/fimmu.2022.908010. eCollection 2022. Front Immunol. 2022. PMID: 35711436 Free PMC article. Review. - Probiotics for preventing acute otitis media in children.
Scott AM, Clark J, Julien B, Islam F, Roos K, Grimwood K, Little P, Del Mar CB. Scott AM, et al. Cochrane Database Syst Rev. 2019 Jun 18;6(6):CD012941. doi: 10.1002/14651858.CD012941.pub2. Cochrane Database Syst Rev. 2019. PMID: 31210358 Free PMC article. - Gut Microbiome as a Possible Cause of Occurrence and Therapeutic Target in Chronic Obstructive Pulmonary Disease.
Lim EY, Song EJ, Shin HS. Lim EY, et al. J Microbiol Biotechnol. 2023 Sep 28;33(9):1111-1118. doi: 10.4014/jmb.2301.01033. Epub 2023 Mar 30. J Microbiol Biotechnol. 2023. PMID: 37164760 Free PMC article. Review.
References
- Food and Agriculture Organization of the United Nations, World Health Organization. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Available at: www.who.int/entity/foodsafety/publications/fs_management/en/probiotics.pdf. Accessed November 2009.
- Pineiro M., Asp N.G., Reid G., Macfarlane S., Morelli L., Brunser O. FAO Technical meeting on prebiotics. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S156–S159. - PubMed
- Vouloumanou E.K., Makris G.C., Karageorgopoulos D.E., Falagas M.E. Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Agents. 2009;34:197. - PubMed
- Weichert S., Schroten H., Adam R. The role of prebiotics and probiotics in prevention and treatment of childhood infectious diseases. Pediatr Infect Dis J. 2012;31:859–862. - PubMed