Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis - PubMed (original) (raw)
Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis
A V Birk et al. Br J Pharmacol. 2014 Apr.
Abstract
Background and purpose: Cardiolipin plays an important role in mitochondrial respiration and cardiolipin peroxidation is associated with age-related diseases. Hydrophobic interactions between cytochrome c and cardiolipin converts cytochrome c from an electron carrier to a peroxidase. In addition to cardiolipin peroxidation, this impedes electron flux and inhibits mitochondrial ATP synthesis. SS-31 (D-Arg-dimethylTyr-Lys-Phe-NH2 ) selectively binds to cardiolipin and inhibits cytochrome c peroxidase activity. Here, we examined whether SS-31 also protected the electron carrier function of cytochrome c.
Experimental approach: Interactions of SS-31 with cardiolipin were studied using liposomes and bicelles containing phosphatidylcholine alone or with cardiolipin. Structural interactions were assessed by fluorescence spectroscopy, turbidity and nuclear magnetic resonance. Effects of cardiolipin on electron transfer kinetics of cytochrome c were determined by cytochrome c reduction in vitro and oxygen consumption using mitoplasts, frozen and fresh mitochondria.
Key results: SS-31 interacted only with liposomes and bicelles containing cardiolipin in about 1:1 ratio. NMR studies demonstrated that the aromatic residues of SS-31 penetrated deep into cardiolipin-containing bilayers. SS-31 restored cytochrome c reduction and mitochondrial oxygen consumption in the presence of added cardiolipin. In fresh mitochondria, SS-31 increased state 3 respiration and efficiency of ATP synthesis.
Conclusions and implications: SS-31 selectively targeted cardiolipin and modulated its interaction with cytochrome c. SS-31 inhibited the cytochrome c/cardiolipin complex peroxidase activity while protecting its ability to serve as an electron carrier, thus optimizing mitochondrial electron transport and ATP synthesis. This novel class of cardiolipin therapeutics has the potential to restore mitochondrial bioenergetics for treatment of numerous age-related diseases.
Keywords: ATP synthesis; Bendavia®; SS peptides; SS-31; Szeto-Schiller peptides; cardiolipin; cardiolipin peroxidation; cyt c; electron transport; mitochondria; mitochondria-targeted peptides.
© 2013 The British Pharmacological Society.
Figures
Figure 1
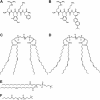
Structures of SS-31, [ald]SS-31, and various phospholipids. (A) SS-31. (B) [ald]SS-31. (C) TOCL. (D) TLCL. (E) POPC. (F) DHPC.
Figure 2
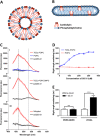
Selective interaction of SS-31 and [ald]SS-31 with CL-containing bilayer membranes and the mitochondrial inner membrane. (A) Diagram of the cross-section through a TOCL-POPC liposome. (B) Diagram of the cross-section through a CL-POPC-DHPC bicelle. (C) The fluorescence emission spectra (in arbitrary units; au) of [ald]SS-31 alone and in the presence of POPC liposomes (upper panel, I), TOCL-POPC liposomes (upper panel, I), TOCL-POPC-DHPC bicelles (middle panel, II), or cyt _c-_depleted mitoplasts (bottom panel, III). Two lines correspond to 535 and 465 nm peaks for [ald]SS-31 alone and in the presence of CL-containing bilayers respectively. (D) SS-31 dose dependently increases turbidity of TOCL-POPC liposomes, but not POPC liposomes. (E) SS-31 increases the turbidity of CL-containing bicelles, but not those containing only PC. All data are shown as means ± SEM, n = 4–6. ***P< 0.001, significant effects of SS-31; Student's _t_-test.
Figure 3
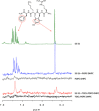
Aromatic amino acids of SS-31 interact with CL. Representative 500 MHz 1H-NMR of the aromatic region (7.6-6.2 ppm). From top, as indicated: (i) SS-31 aromatic protons of phenylalanine (7.3 ppm) and dimethyl-tyrosine (6.5 ppm). (ii) POPC-DHPC bicelles do not substantially affect aromatic proton peaks; (iii) POPC does not contribute to the spectrum in the aromatic region. (iv) TOCL-POPC-DHPC bicelles remodel and broaden the aromatic protons of SS-31. (v) TOCL-POPC-DHPC bicelles themselves contribute no peaks in this range.
Figure 4
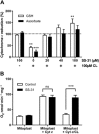
SS-31 promotes electron transfer through the cyt _c_-CL complex. (A) SS-31 promotes reduction of cyt c in the presence of CL. Dotted line represents reduction (%) of cyt c with either GSH or ascorbate. 100 μM SS-31 does not promote reduction of cyt c in its native conformation, without CL. In the presence of CL, SS-31 dose dependently rescues reduction of cyt c. **P<0.01. ***P<0.001, significantly different from ‘no CL’ values; one-way ANOVA with Dunnett's multiple comparison test. (B) SS-31 promotes oxygen consumption in mitoplasts where cyt c is inhibited by excess CL. SS-31 did not affect TMPD/ascorbate-induced oxygen consumption in cyt _c_-depleted mitoplasts, in the presence or absence of exogenous cyt c. When exogenous cyt _c_-activated oxygen consumption was inhibited by CL, SS-31 completely restored respiration. All data are shown as means ± SEM, n = 4–8. ***P<0.001, significant effects of SS-31 on respiration in mitoplasts; Student's _t_-test.
Figure 5
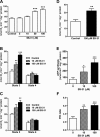
SS peptides promote mitochondrial oxygen consumption and ATP synthesis. (A) Electron flux was measured as oxygen consumption in frozen mitochondria, where SS-31 dose dependently increased oxygen consumption. (B) SS-31 dose dependently increases O2 consumption during state 3 respiration in the presence of complex I substrates (glutamate/malate). (C) SS-31 dose dependently increases oxygen consumption during state 3 respiration in the presence of complex II substrate (succinate). (D) SS-31 promotes oxygen consumption after direct reduction of cyt c by TMPD/ascorbate. (E) SS-31 dose dependently promotes ATP synthesis during state 3 respiration in the presence of succinate. (F) SS-31 dose dependently increases P/O ratio during state 3 respiration in the presence of complex II substrate (succinate). Data are shown as means ± SEM, n = 5–10. * P< 0.05, ** P< 0.01, *** P<0,001, significantly different from control; one-way
anova
with Dunnett's multiple comparison test (A, B, C, E) or Student's _t_-test (D).
Figure 6
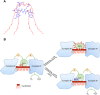
(A) Proposed model of the 1:1 interaction of SS-31 with CL shows favourable overlap of opposite electrostatic charges and hydrophobic regions. (B) Schematic diagram of mitochondrial dysfunction during early post-ischaemia. Unfolding of cyt c due to hydrophobic interaction with CL on the outer leaflet of the inner membrane prevents electron transport through complex III and IV. SS-31 localizes to CL, stabilizes cyt c in its folded conformation and restores electron transport.
Similar articles
- First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics.
Szeto HH. Szeto HH. Br J Pharmacol. 2014 Apr;171(8):2029-50. doi: 10.1111/bph.12461. Br J Pharmacol. 2014. PMID: 24117165 Free PMC article. Review. - The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin.
Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. Birk AV, et al. J Am Soc Nephrol. 2013 Jul;24(8):1250-61. doi: 10.1681/ASN.2012121216. Epub 2013 Jul 11. J Am Soc Nephrol. 2013. PMID: 23813215 Free PMC article. - Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis.
Liu S, Soong Y, Seshan SV, Szeto HH. Liu S, et al. Am J Physiol Renal Physiol. 2014 May 1;306(9):F970-80. doi: 10.1152/ajprenal.00697.2013. Epub 2014 Feb 19. Am J Physiol Renal Physiol. 2014. PMID: 24553434 - A mitochondria-targeted antioxidant can inhibit peroxidase activity of cytochrome c by detachment of the protein from liposomes.
Firsov AM, Kotova EA, Orlov VN, Antonenko YN, Skulachev VP. Firsov AM, et al. FEBS Lett. 2016 Sep;590(17):2836-43. doi: 10.1002/1873-3468.12319. Epub 2016 Aug 4. FEBS Lett. 2016. PMID: 27442781 - Cardiolipin-targeted peptides rejuvenate mitochondrial function, remodel mitochondria, and promote tissue regeneration during aging.
Szeto HH, Liu S. Szeto HH, et al. Arch Biochem Biophys. 2018 Dec 15;660:137-148. doi: 10.1016/j.abb.2018.10.013. Epub 2018 Oct 23. Arch Biochem Biophys. 2018. PMID: 30359579 Review.
Cited by
- Natural history comparison study to assess the efficacy of elamipretide in patients with Barth syndrome.
Hornby B, Thompson WR, Almuqbil M, Manuel R, Abbruscato A, Carr J, Vernon HJ. Hornby B, et al. Orphanet J Rare Dis. 2022 Sep 2;17(1):336. doi: 10.1186/s13023-022-02469-5. Orphanet J Rare Dis. 2022. PMID: 36056411 Free PMC article. Clinical Trial. - TAZ encodes tafazzin, a transacylase essential for cardiolipin formation and central to the etiology of Barth syndrome.
Garlid AO, Schaffer CT, Kim J, Bhatt H, Guevara-Gonzalez V, Ping P. Garlid AO, et al. Gene. 2020 Feb 5;726:144148. doi: 10.1016/j.gene.2019.144148. Epub 2019 Oct 21. Gene. 2020. PMID: 31647997 Free PMC article. Review. - Role of abnormal energy metabolism in the progression of chronic kidney disease and drug intervention.
Liu X, Du H, Sun Y, Shao L. Liu X, et al. Ren Fail. 2022 Dec;44(1):790-805. doi: 10.1080/0886022X.2022.2072743. Ren Fail. 2022. PMID: 35535500 Free PMC article. - Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure.
Dey S, DeMazumder D, Sidor A, Foster DB, O'Rourke B. Dey S, et al. Circ Res. 2018 Jul 20;123(3):356-371. doi: 10.1161/CIRCRESAHA.118.312708. Epub 2018 Jun 13. Circ Res. 2018. PMID: 29898892 Free PMC article. - Role of Lysocardiolipin Acyltransferase in Cigarette Smoke-Induced Lung Epithelial Cell Mitochondrial ROS, Mitochondrial Dynamics, and Apoptosis.
Bandela M, Suryadevara V, Fu P, Reddy SP, Bikkavilli K, Huang LS, Dhavamani S, Subbaiah PV, Singla S, Dudek SM, Ware LB, Ramchandran R, Natarajan V. Bandela M, et al. Cell Biochem Biophys. 2022 Mar;80(1):203-216. doi: 10.1007/s12013-021-01043-3. Epub 2021 Nov 1. Cell Biochem Biophys. 2022. PMID: 34724158 Free PMC article.
References
- Bayir H, Tyurin VA, Tyurina YY, Viner R, Ritov V, Amoscato AA, et al. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Ann Neurol. 2007;62:154–169. - PubMed
- Bode AM, Miller LA, Faber J, Saari JT. Mitochondrial respiration in heart, liver, and kidney of copper-deficient rats. J Nutr Biochem. 1992;3:668–672.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources